- Home
- >
- Malaysia
- >
- Cosmetics
Cosmetics Product Regulatory Affairs Services in Malaysia
Our team helps cosmetic brands handle product registration in Malaysia with NPRA. We manage the full regulatory process so you can focus on bringing your products to market faster and with confidence.
Malaysia NPRA Support for Cosmetics Product Registration
Artixio is a leading consulting company with presence in over 100+ countries, helping our clients with effective regulatory strategies to successfully commercialize their products in the Malaysian market. With our regulatory experts team, we offer complete support right from product development up to its successful launch and then post-approval maintenance in compliance with Malaysian regulatory requirements.
Overview of Cosmetics in Malaysia
Artixio provides a step-to-step guidance for all submission for notification of the cosmetics in Malaysia, and it is done through the Online QUEST system via NPRA’s website in compliance with the NPRA regulatory requirements.
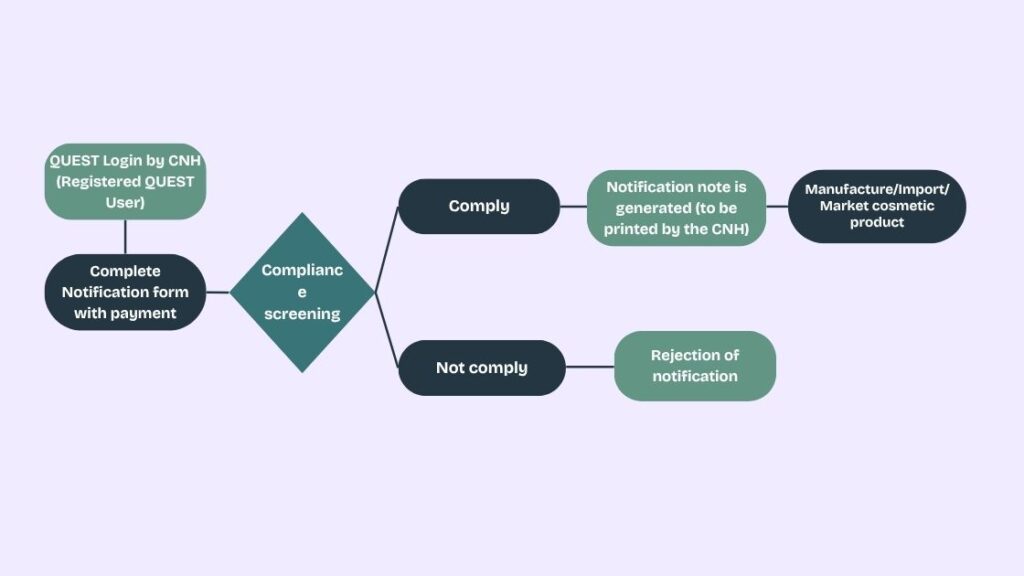
Process for Cosmetic notification in Malaysia
A notification number is unique for each product and its variant (if any). CNH is responsible for ensuring the maintenance of valid notification number for products in the market. Existing notification number is retained for product notification renewed before the expiry date. For products that are not renewed within the given timeline and products with changes requiring new notification, a new notification number will be generated.
Fees
Validity period
Certificate of Free Sale (CFS)
- A CFS is a document which states that the product can be freely sold in Malaysia. It is not a mandatory requirement.
- It will be issued by the NPRA upon request by the CNH willing to export their notified cosmetic product to another country that requires the certificate.
Artixio Offers the Following Services for Cosmetic Products in Malaysia
- Stepwise guidance for QUEST registration
- Advice on data requirements
- Strategies to get the cosmetic product successfully notified
- Support throughout the product commercialization journey
- Post-approval maintenance of the product
Why Choose Artixio For Cosmetic Product RA Services In Malaysia?





Regulatory Support - Planning & Execution for Malaysia
FAQs
Which is language is required for documents submission for cosmetic products to NPRA Malaysia?
What is the processing fees for renewal of notification of a cosmetic product in Malaysia?
How to submit changes to an existing cosmetic product notification in Malaysia?
| Type of Change | Description | Fees | Notification Number |
|---|---|---|---|
| Type 1 Change | Changes that only require amendments to the current notification. | No charge required | Notification number remains the same. |
| Type 2 Change | Changes that require a new notification. | Processing fee RM 50.00 | New notification number will be issued to the product. |
How to obtain a Certificate of Free Sale (CFS) in Malaysia?
Related Malaysia Services
Regulatory Expertise Across
Multiple Countries












Blogs

Registration of Biologics in
Biologics have emerged as a significant treatment for various diseases in Malaysia. With their...

Pharmaceutical Regulations and Registration
Before placing a pharmaceutical product on the Malaysian market, it’s important to understand how...

NPRA Health Supplements Regulations
In Malaysia, it’s not unusual to find health supplements being sold online without proper...