Regulatory Outsourcing Services for Life Sciences
Artixio, a global regulatory solutions and services company, has been helping life sciences companies from around the world build their operations teams in India. Many of these companies had no prior presence in the country and limited experience with the local landscape.

Services We Offer
Are you looking for…
- Expanding your global presence?
- Growing your operations team?
- Improving operations cost efficiency and increasing profitability?
- Minimize your initial investment risks by ‘testing the waters’ first?
- A partner with right experience, resources to help you achieve these goals?
Our Talent Pool
Includes professionals with experience ranging from 2 to 20 years, covering roles from associate level to senior management. As a global regulatory solutions and services provider, Artixio supports companies with skilled resources that fit their operational and compliance needs.
Regulatory
- Regulatory Affairs
- eCTD Publishing and Submission
- Regulatory Labeling
- Artwork Proofreading
- Graphic Design
- Project Management
- Regulatory Information Management
- Change Coordination
- Medical Writing
Market Intelligence
- Secondary Market Research
- Literature Review
- Primary Market Research
- Competitive Intelligence
- Report Writing
- Aggregate report scheduling
- Adhoc reports
Medico-Marketing
- Social Media Listening & Review
- Content Writers and Content Management
- Social Media Managers
Artixio as Your Regulatory Outsourcing Partner

Secured IT access for global operations enabled for document sharing and remote working

24x7 operations enabled using robust IT infrastructure enabling data security complying with global standards

Our team have built, operated and transferred operations and commercial team in India managing global projects from centralized location

We understand the process in India and have access to resources for setting up teams and organizations right from research, strategy. incorporation, set up and execution

Right from senior experts to junior professionals across functions in regulatory affairs, market research, R&D, we have access to wide pool of resources available to be onboarded

Start small with Artixio by deploying a small team for pilot and scale to build a complete organization around

Our inhouse team includes diverse experts from R&D, Commercial, Regulatory, Legal, Operations, Project Management, IT and HR services to guide our partners through entire. process

We help reduce operational costs and improve efficiency without compromising quality.

With experience across multiple process of different complexities and scale, we have proven operational models to drive excellence based on SOPs, KPIs and metrics, organization structure to manage the process.
Why Choose India for Your Global Regulatory Outsourcing?
- India is world’s 3rd largest pharmaceutical industry by volume
- India is largest provider of generic medicines globally with supply to over 200 countries and territories
- Among largest workforce in India for IT and pharmaceutical industry with proficiency in English language
- Qualified and experienced lifesciences professionals across Regulatory, R&D and other Knowledge Management industries
- Opportunity to manage global operations from centralized team
How it Works?
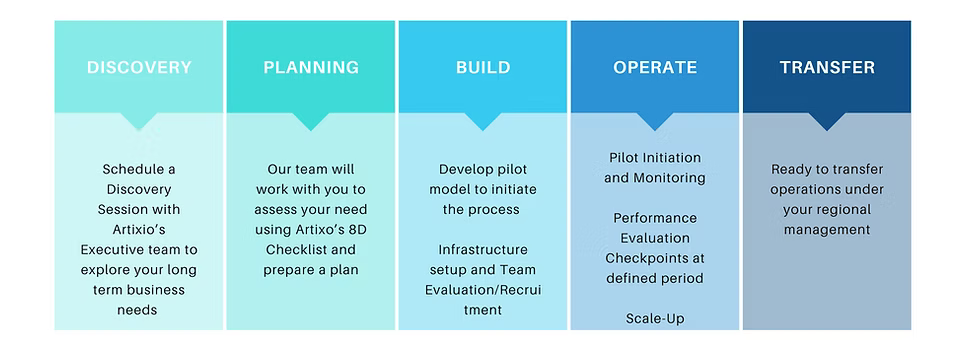
Still Have Questions ?
Specialized Regulatory
Services Across Multiple Industries
Expert Regulatory Services To Streamline Compliance

Regulatory Intelligence & Strategy

Medical & Technical Writing

Publishing & Submission






Regulatory Affairs Across Multiple Countries

India

Singapore

Mexico

Brazil

Vietnam

Malaysia

Argentina

Colombia

Taiwan

China

European

Thailand

Indonesia

Philippines

USA

Japan

Qatar

South Korea
Tips & Articles

Strategic Criteria for Selecting
All industries in Thailand, including cosmetics, food, medical devices, and nutrition, develop very fast,...

Combinational Drugs Approval Process
The combinations in Thailand play a major role in marking the pharmaceutical development. Combinational...
Thailand Government Schemes for
Pharmaceutical and Medical device industries in Thailand are growing to meet the increasing demand...
