The increasing demand for organic, safe, and cruelty-free cosmetics has promoted the production and sale of cosmetic products in Argentina. These cosmetic products are regulated by the ANMAT (Administración Nacional de Medicamentos, Alimentos y Tecnología Médica) which is the main regulatory authority in Argentina. Argentina serves as a good scope for the development of domestic and foreign cosmetic products.
Now, let’s look down deep into the aspects of cosmetic products in Argentina.
Definition Of Cosmetics By ANMAT:
In Argentina, cosmetics products are defined as- “Any mixture or substance which is intended to apply superficially in contact with parts of the human body (capital and hair system, epidermis, lips, nails, external genital organs, teeth, and oral mucous membranes) for the sole purpose of cleaning, modifying appearance, perfuming, odor removing and to keep or protect them in good condition”.
Argentina Cosmetics Market Overview
- Argentina Beauty and Personal Care Products Market is expected to register CAGR of 5.26% during the forecast period 2025-2030.
- Baby care products market in Argentina attained a the market size of US 41,776.559 million in 2026.
Cosmetics Classification by ANMAT
The cosmetic products in Argentina are categorized by ANMAT in association with the Mercosur guidelines for the confirmation of safety, restrictions for use and mode, efficacy of products as written in provision No. 345/06 (ANMAT) and Resolution No. 07/05.
The table below gives an overview of the classification of cosmetic products in Argentina and its regulatory requirements.
| Category | Risk-level | Regulatory Requirements | Examples |
| Grade 1 | Low-risk level | Grade 1 cosmetics involve simplified regulations.
Dossier data is not required. Documents such as safety data, efficacy data, toxicology studies, etc. are not required. Nominal audits and inspections are conducted for grade 1 products. |
Nail Polishes
Lipsticks Shampoo Lotions |
| Grade 2 | High-risk level | Grade 2 cosmetics involve detailed regulations.
Detailed documents are required for toxicology studies, efficacy studies, and safety data. It requires a detailed dossier submission. Comprehensive audits and inspections are conducted for grade 2 cosmetics. |
Sunscreens Hair-dyDeodorantsts Eye-area products |
Grade I
They are the products used for personal hygiene, perfuming, and cosmetic purposes whose formulation meets the condition of GMC Res. No. 110/94 and are characterized by having basic properties or elemental properties. Initially the verification is not necessary and does not require detailed information as to their restrictions on use or mode, due to the intrinsic character of the product mentioned in the indicative list given in Annex II.
Grade II
Products use for personal hygiene, cosmetics and perfumes whose formulation falls within the definition of GMC Res. No. 110/94 have specific indications and their characteristics require verification on the product safety, efficacy, care, information, modes and restriction for use as mentioned in Annex III.
This classification was based on the probability of occurrence of undesirable effects due to improper use of product, purpose for use, its formulation, parts of the body to which it is intended and care to be perceive in is use.
Regulatory Parties In Argentina
- Ministry Of Health
- ANMAT
In Argentina, ANMAT- National Administration of Drugs, Food and Medical Technology handles the regulation of cosmetics through Resolution MS and AS No. 155 of 1998.
ANMAT updates the standards related to cosmetic products for Personal Hygiene, perfuming and the activities intrinsic to them. – BO 04/02/98
- MERCOSUR
ANMAT is harmonized with the European Union or southern Common Market (el Mercado Comun del sur, MERCOSUR is a trade association of five countries: Brazil, Argentina, Uruguay, Paraguay and Venezuela in which Argentina is a part of MERCOSUR.
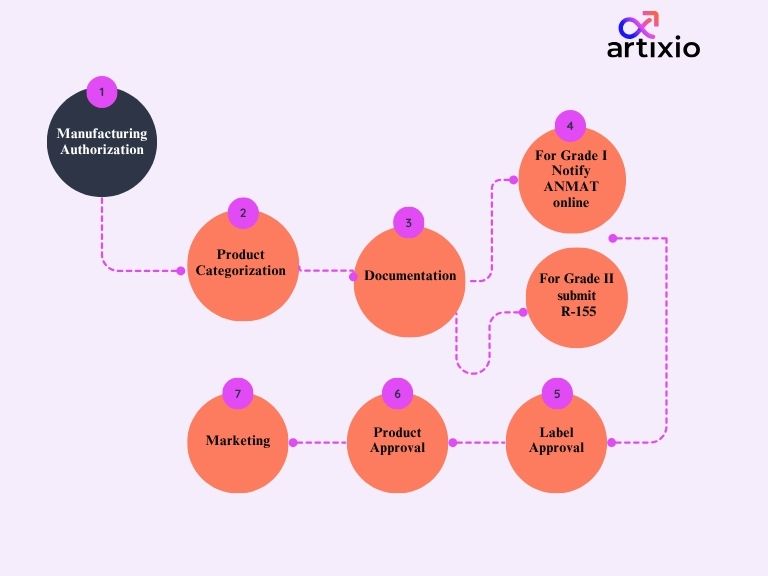
Cosmetics Product Registration Process In Argentina
Grade I Products Registration In Electronic File:
Manufacturers or importers of cosmetics authorized by ANMAT can make the admission of cosmetic products online. IT department of ANMAT grants User name and personal access Key by which the company can enter the cosmetics system. For establishment of that company has to fill out two forms namely-
- Application for registration of initiation of electronic file system for cosmetic products.
- Registration request of the activities in the electronic file system for cosmetic products.
These forms are submitted at the entrance desk of Health Products Surveillance Directorate for addressing the inspection service of cosmetic products. After successful inspection, ANMAT computer coordination will deliver the password and username.
Grade II Products Registration In Electronic File:
The following are the regulatory requirements for grade II registration in electronic file:
- Firstly, the R-155 form should be submitted for the registration of your grade II cosmetic.
- A product and technical requirement list should be submitted which includes all the details of the safety, composition, and function of the cosmetic products.
- A modification request form is submitted if there is any change in the product name, label, composition, etc.
- An Alternative Establishment Request Form should be submitted for a change in cosmetic manufacturing location.
- For product manufacturer change, a change of producer establishment form should be submitted.
- Formula change request form is submitted if there is a change in the concentration or ingredients formula used in the cosmetic preparation.
Cosmetics Registration Documents Requirements in Argentina:
Following is the list of documents required by ANMAT for the registration of cosmetics in Argentina:
- Application Form R-155
- GMP Certificate
- Product Technical Sheet
- Cosmetic Ingredient List
- Manufacturing Authorization
- Product Label Draft
- Payment Receipt
Import Of Cosmetic Products In Argentina
The import of cosmetics in Argentina can be costly and quite difficult. There are agencies which give favor for the importation of cosmetics from Argentina. One of the leading countries in the beauty and cosmetics production in Latin America is Argentina.
Documents required for import cosmetics are –
- Product import authorization request form.
- Payment receipt for the corresponding fee.
- Copy of certificate of registration showing qualification of cosmetics import establishment.
- Copy of enabling provision of the formation having the data of recent technical director.
- Copy of the designation of a technical director other than the one designated in the enabling provision.
- Copy of the demonstration made to the health authority of the cosmetics product involved along with the details of data of product, owner of product, and importing establishment are recorded.
- Affirmation required by ANMAT disposition 162/01.
- Copy of letter of representation.
- Document of transport.
Certificate Of Free Sale (CLV)
Grade I
Application – The request is made through PLATFORMA DE TRAMITITES A DISTANCIA (TAD). For this the applicant company must be registered in the electronic file of ANMAT.
Obtaining certificate – after the application has been evaluated, the CLV may be issued or rejected. In both situations notification will be given via TAD. Also the company must enter its “notifications” that is the certificate should no longer be withdrawn personally.
Grade II
Application – The request is made through PLATFORMA DE TRAMITES A DISTANCIA (TAD) which is approved by decree no. 1063/2016 and should be regulated by Resolution no. 12/2016 of SECRETARIA DE MODERNIZACION ADMINISTRATIVA of the Ministerio de Modernización de la Nación and Resolution no. 73- E / 2017 of the Ministerio de Modernización de la Nación.
Obtaining certificate – For obtaining the certificate issued electronically through TAD, notification is sent by mail to the company. The person responsible will enter the platform and search the Notifications tab and download the certificate and cited with that which are the part of the electronic file.
Correction of the procedure – If there are any errors in the application, or the authority need some more information, the Health Authority will carry out a correction process.
Labelling Of Cosmetic Products
Labeling should be in Spanish language and should be in accordance with the Resolution GMC No. 36/04. The requirements in labeling are given below:
- Identity of the product in the language of the state where the product is going to be sold.
- Statement providing net quantity in metric units of measurement.
- Any warnings or cautions.
- Name and address of the manufacturer or distributor.
- Directions given for the use of product.
- Ingredient list ( using INCI ).
- Expiration or use before the date.
Animal Testing
In Argentina a bill is proposed to promise the end of animal testing for cosmetics. The bill was introduced by Rio Negro province Senator and Magdalena Odrada. If this bill is made into law will prohibit the sale of new animal tested cosmetics and the animal testing for cosmetic ingredients.
Cruelty free cosmetics may be the further trend for the sale of cosmetics in Argentina.
Challenges:
The regulatory procedures for cosmetics may face some challenges such as:
- Complex regulatory requirements especially for grade II cosmetics products.
- Special requirements for imported cosmetics may pose a challenge for foreign manufacturers.
- The lack of an online documentation process may cause a delay in the approval process.
- The high cost of the whole regulatory procedure may also hamper the approval process.
- Sometimes, it becomes difficult to keep the cosmetic product in compliance with the frequent guideline Changes of Mercosur and ANMAT.
So, for complete assistance for your cosmetic product regulatory compliance and speedy approval process partner Artixio. Our specialized local team with regulatory intelligence-driven solutions helps make your cosmetic product successful in the Argentina market.
For more information contact info@artixio.com
FAQs- Cosmetic Regulations In Argentina
1. How are cosmetics classified in Argentina?
They are categorized according to requirements for the confirmation of safety, restrictions for use and mode, efficacy of products in Grade I and Grade II.
2. Who regulates cosmetics in Argentina?
In Argentina, ANMAT- National Administration of Drugs, Food and Medical Technology handle the regulation of cosmetics through Resolution MS and AS No. 155 of 1998.
3. What is Argentina’s ANMAT harmonized with?
ANMAT is harmonized with the European Union or southern Common Market (el Mercado Comun del sur, MERCOSUR) is a trade association of five countries: Brazil, Argentina, Uruguay, Paraguay and Venezuela in which Argentina is a part of MERCOSUR.
4. What is Product Registration Process in electronic file in Argentina?
Manufacturers or importers of cosmetics authorized by ANMAT can make the admission of cosmetic products online. IT department of ANMAT grants Username and personal access Key by which the company can enter the cosmetics system and follow the steps. It comes in the Grade I type.
5. Through which platform is the request made for application certificate of free sale in Argentina?
The request is made through PLATFORMA DE TRAMITITES A DISTANCIA (TAD).
6. What are the requirements for labelling of cosmetic products in Argentina?
Most importantly, Labeling should be in Spanish language and should be in accordance with Resolution GMC No. 36/04 along with other requirements.
7. Is cosmetic business profitable in Argentina?
Argentina Beauty and Personal Care Products Market is expected to register CAGR of 7.21% during the forecast period 2020-2025.
8. In what language are the approval documents submitted in Argentina?
All the documents for cosmetic approval in Argentina should be submitted in Spanish.
9. What is the time span required for cosmetic product approval in Argentina?
The grade I cosmetics are approved immediately whereas grade II cosmetics approval takes 3-6 months.