Biologics and Biosimilars hold great therapeutic value, as they are the emerging class of pharmaceuticals products. They hold the potential for treating many life-threatening conditions. In China, these biologics and biosimilars are regulated by the National Medical Products Authority (NMPA).
This blog gives a detailed overview of the regulatory requirements of NMPA for biologics and biosimilar approval.
Overview Of The National Medical Products Authority (NMPA):
- The NMPA was formed in the year 1950 in China for the supervision of drugs.
- Before it was known as China Food and Drug Administration and State Food and Drug Administration.
- In March 2013 this authority was named National Medical Products Authority.
- The main responsibility of the NMPA includes the smooth and safe regulation of pharmaceutical products in China.
- The Center of Drug Evaluation (CDE), which is a part of NMPA, is responsible for the application review of biologics and biosimilars.
- The biosimilars should comply with all the guidelines mentioned under the Technical “Guidelines for the Development and Evaluation of Biosimilars” by the NMPA.
- The NMPA accepts the approval request for the biosimilar and biologics through its online portal.
- The NMPA usually takes 12-18 months for the application review and its approval, however, the biosimilars may take less time for its approval.
NMPA Classification of Biologics and Biosimilars in China
Under the regulations for Biologics and Biosimilars in China, the products are classified into Preventive or Therapeutic or IVD Reagent Products. For the purpose of registration, each category is further classified into different sub-categories –
| Preventive Biologics | Therapeutic Biologics | IVD Reagent Products |
| Vaccine Biologics – Immunization and non-immune planned vaccines for prevention and control of disease and prevalence | Used in treatment Eg – Proteins, peptides, derivatives from engineered cells |
Eg – Blood sourcing screening IVD reagents labeled with radionuclides |
| Innovative vaccines – Not available globally
Improved Vaccines – Listed in China or global Health agencies and improvements have been made for better quality, safety or efficacy and offer significant advantages Domestic or Overseas Listed vaccines |
Innovative Biological Products- Not available globally
Improved Biological Products- listed in China or global Health agencies and improvements have been made for better quality, safety or efficacy and offer significant improvement in treatment outcomes Domestic or Overseas Listed vaccines |
Innovative IVD reagents
IVD reagents listed in China or global Health agencies |
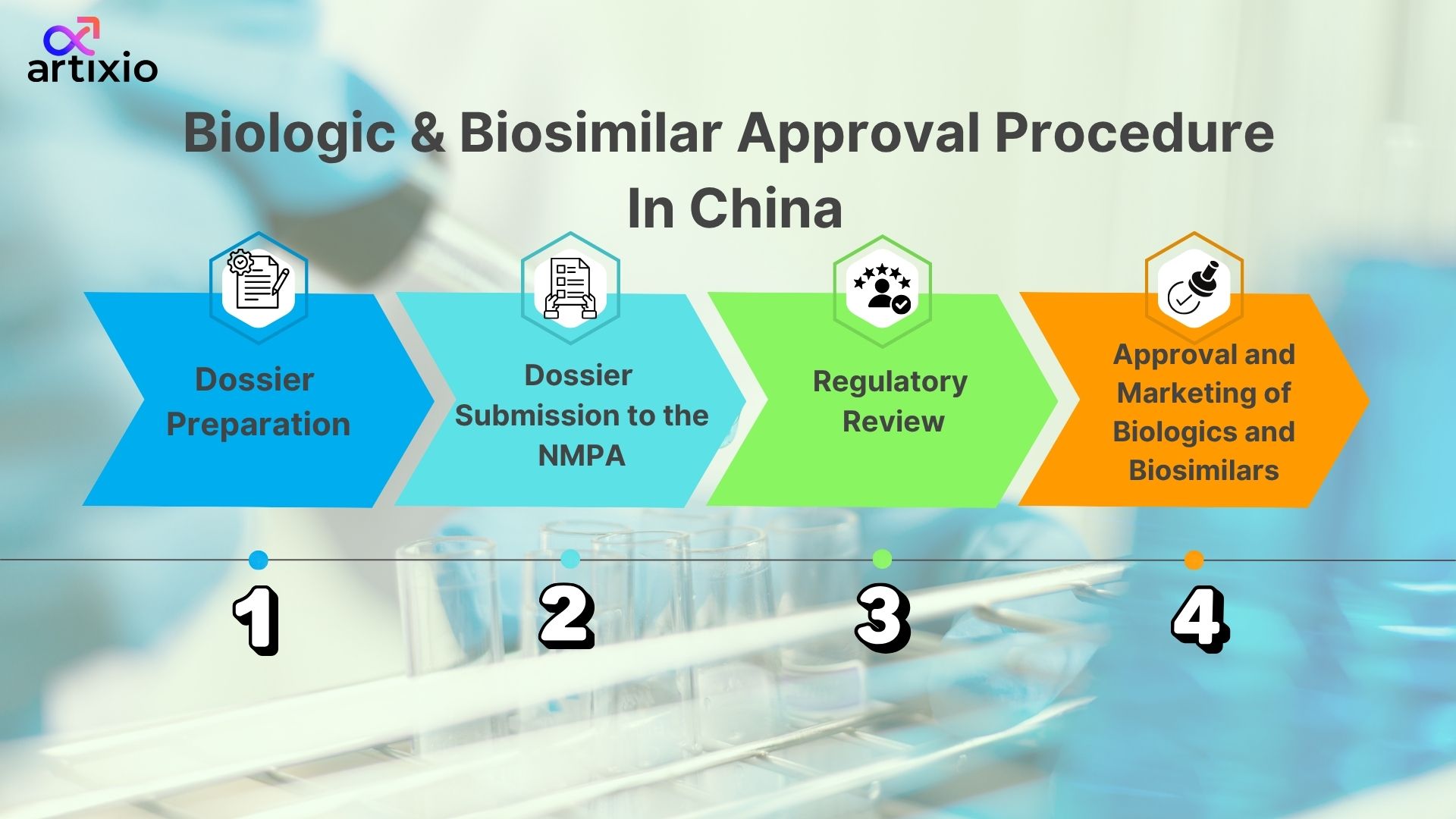
Regulatory Process For Biologics & Biosimilars In China:
1. Dossier Preparation:
A detailed dossier is prepared that includes all the details of biologic or biosimilar products including its pre-clinical and clinical trials, manufacturing details, quality control and quality assurance details, etc.
2. Dossier Submission to the NMPA:
The dossier prepared is checked and then submitted to the NMPA along with the Biologic License Application (BLA) through the online platform of the Center of Drug Evaluation (CDE) under the NMPA.
3. Regulatory Review:
The NMPA then reviews the application along with all the details in the dossier file and decides whether to approve the biologic within 12-18 months, whereas for biosimilar it takes less time depending upon its similarity with the biologic.
4. Approval and Marketing:
Once the approval is received from the NMPA, the biologic or biosimilar can be sold in the Chinese market. Then the product is given the trade name before it’s sold.
Required Documents by NMPA:
Following is the list of the main documents required for biologics and biosimilar approval:
Manufacturing unit document:
- Business License
- GMP certificate
- Authorization letter
Chemistry, Manufacturing, and Controls (CMC documents):
- Drug detail report
- Batch record
- Certificate of Analysis
- Product stability report
Pre-clinical data report:
- Pharmacology report
- Toxicology report
Clinical data report:
- Phase studies report
- Bioequivalence study report
Packaging and labeling data report
CFS (for imported product)
Fee Structure for Biosimilar & Biologics in China:
The fees associated with the approval of biosimilar and biologics drugs involve mainly the application fees, which are lesser for biosimilar products as compared to biologics.
For small manufacturing companies, the fee may get lower as they are applicable for discounts.
The table given below gives an idea about the fee structure for biologics and biosimilars in China:
| Product Type | Application Fee (approx.) | Key Points |
| Biologic | $35,000 |
The amount may increase if large clinical data is present.
|
| Biosimilar | $17,500 |
The review period is less, and the fee involved may also get less depending upon the similarity with the biologic.
|
Post-Marketing Surveillance:
- Post-marketing surveillance is conducted to assess the safety of drug products after their sale among the patients.
- The PMS should be conducted for biologic products and its report should be submitted to the NMPA annually.
- The monitoring of adverse reactions (ADRs) is necessary through the hospital records.
- On serious adverse effect occurrence, the biologic or biosimilar product should be re-evaluated.
- However, the report is submitted annually but the monitoring should be frequently done by the manufacturer to prevent and address any product safety issues.
Conclusion:
China is becoming the global player in the pharma market with its emerging developed biologics, which have proved to be a boon in chronic pharma disease treatment. Thus, this opens the scope for both domestic as well as foreign manufacturers to develop their biological products in China and bring their products to the Chinese market with the simple and time-efficient procedures of the NMPA.
So, if you intend to bring your biologic or biosimilar product into the Chinese pharma market and struggling to figure out the regulatory procedure of NMPA, partner with Artixio, where we aim to make your regulatory journey simply efficient, avoid regulatory delays, and help you make your product successful.
For any queries reach out at info@artixio.com
FAQs:
How can foreign manufacturers bring their biologic to the Chinese market?
Foreign manufacturers can bring their biologic into the Chinese market through direct marketing authorization, technology transfer, or by collaborating with domestic companies.
Which are the biological products in high demand in China?
Biologics used for cancer, diabetes, and autoimmune treatment are in high demand in China.
Does China have any schemes to support and promote the development of biologics and biosimilars?
Yes, China has launched schemes such that provides incentives, tax-benefits, etc. to support and promote the development of biologics and biosimilars. Some of these schemes are Made in China 2025 and Healthy China 2020.
Is local manufacturing of biologics mandatory in China?
No, but local manufacturing of biologics is promoted as it helps in the regulatory procedures and reduces the import cost etc.