A Biological specimen is a pharmaceutical product prepared by utilizing living cells and tissues to prevent, diagnose, or treat diseases. The biological specimen products include vaccines, recombinant products, sera, etc. In India there are various government bodies such as Indian Council of Medical Research (ICMR), Directorate General of Foreign Trade (DGFT), Department of Biotechnology (DBT), etc. that regulate the biologic specimen to ensures its safety, efficacy and safety.
Thus, to bring a biological specimen in India the manufacturer should take care and comply with the regulations and documents requirement so avoid non-compliance from the regulatory authorities in India.
Import of Biological Specimen:
Importing biological specimens into India is a critical process for various sectors, including medical research, pharmaceutical development, and diagnostic testing. Ensuring compliance with Indian regulations is essential to avoid legal and logistical challenges. This blog outlines the key regulations and necessary approvals for importing biological specimens into India.
If you need reliable Biologics Regulatory Affairs Support in India, our team at Artixio can help you navigate every step with clarity and confidence.
Regulatory Authorities Responsible For Import of Biological Specimen:
Indian Council of Medical Research (ICMR):
- ICMR regulates the import and use of biological specimens in India.
- It looks that all the guidelines are strictly followed by the biological specimen.
Directorate General of Foreign Trade (DGFT):
- It regulates the import and export of biological specimens in India.
- DGFT takes care of the compliance of rules for the export and import of biological specimens.
CBIC – Central Board of Indirect Taxes and Customs:
- CBIC regulates the import and custom clearance domains for biological specimens.
Regulatory Process For Import Of Biological Specimen:
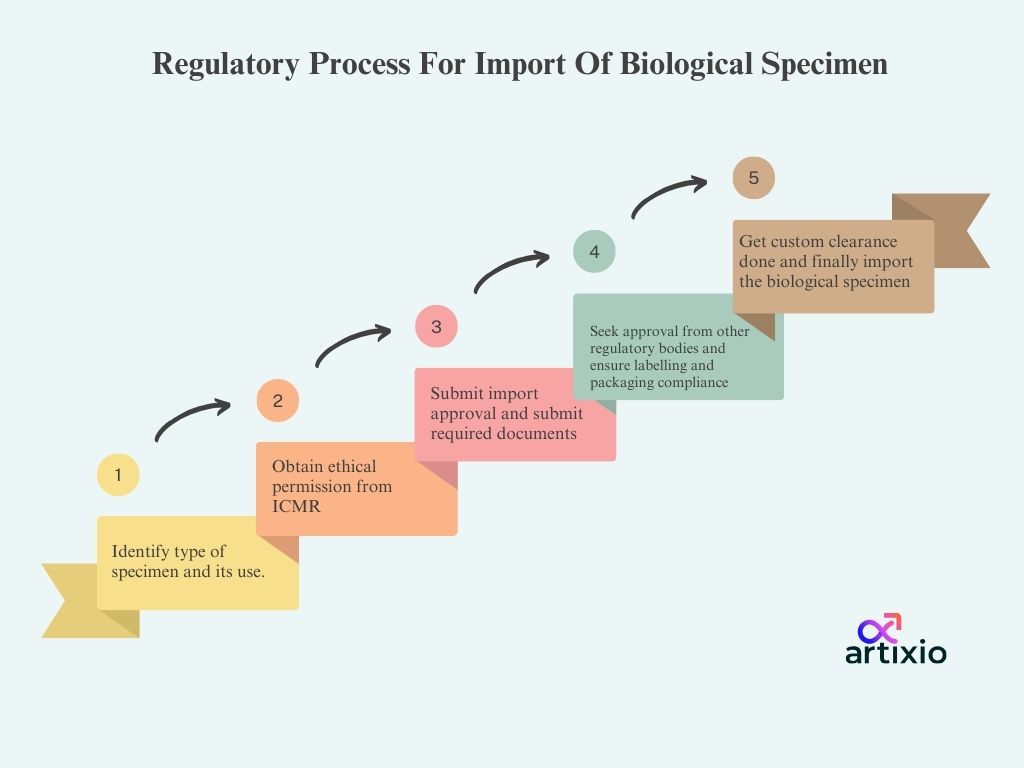
The import of biological specimen process begins with identification of specimen type and its purpose of importation, then ethical approvals are taken from ICMR after which import permission application with attaching relevant documents is submitted to the DGFT, after which permission from other regulatory bodies is also taken such as NBA, DGA,etc. Then the packaging and labelling compliance are confirmed and after the custom clearance the biological specimen is imported.
Types of Biological Specimens
Biological specimens that may be imported include, but are not limited to:
Products derived from live tissues or cells, such as vaccines, sera, and recombinant products,
- Blood and blood components
- Tissue samples
- Microbial cultures
- DNA/RNA samples
- Other human or animal biological materials
Biological Sample Examples:
The biological sample examples include the following:
- Blood sample
- Urine sample
- Sputum sample
- Saliva sample
- Cerebrospinal fluid sample
- Cell sample
- Genetic material sample
- Amniotic fluid sample
- Pleural fluid sample
Purpose of Importing Biological Specimens into India
Biological specimens can be imported for various purposes, including:
- Clinical research
- Diagnostic purposes
- Therapeutic uses
- Pharmaceutical development
- Academic research
- Lab analysis
- R & D testing
Ethical Considerations For Biological Specimens:
While collecting biological specimens there are a certain set of ethical considerations that should be followed. They are as follows:
- Obtaining informed consent
- Maintaining patient confidentiality
- Clear expression of specimen collection to the patient
- Minimization of risk during collection
- Samples should be used only for the purpose for which the donor is agreed.
Outdated Regulation For Biological Specimen In India:
Prior approval for the biological specimen was mandatory as this process was quite tedious and time-consuming. The approval took almost 4-5 months which prolonged the regulatory and diagnostic processes. This created a lot of delays in the further specimen procedures and caused a loss of revenue. The complete regulatory procedure took almost a year. Hence some changes to the guidelines were made.
Amended Regulation For Biological Specimen In India:
The new amendments in the guidelines removed the compulsion of import-export license requirements for biological specimens.
- The biological specimen can be exported or imported without any prior notification to the government authorities. This made the whole process time-efficient and convenient for the companies and researchers.
- The changes to the guidelines were made by the ABLE team under the leadership of Honorary Chairperson, Dr. Kiran Mazumdar Shaw, Founder of Biocon.
- These changes were brought to notice by the DGFT on 4th August 2016 under the Notification number 19/2015-2020 by the director general of DGFT, Mr. Anup Wadhawan.
- According to the new amendments the researchers or company head needs to file a self-attested certificate for the customs authority assuring the safety, efficacy, and quality of the biological specimen.
Hence, now the biological specimen can be exported or imported at a faster rate without prior approval or notification to the government bodies.
Conclusion:
The biological specimen importation in India is thus a critical process where compliance from many regulatory bodies is mandatory, only after which the biological specimen can be imported in India. Thus, the biological specimen should study all the rules and regulations in depth to ensure its compliance.
For any regulatory assistance for importing, your biological specimen in India contact Artixio , as we have specialized team to assist you throughout your regulatory journey.
If you’re planning to import or work with biological specimens in India, expert regulatory guidance is essential. Our Biologics Regulatory Affairs Consultants in India ensure compliance and seamless approvals. Contact info@artixio.com today!
FAQs:
1. How are biological specimens preserved for testing?
The preservation of biological specimens is mandatory for accuracy in the results. The common preservation techniques involve refrigeration, freezing, and use of preservation such as alcohol.
2. What are some of the precautions to be taken while handling biological specimens?
Some of the precautions to be taken while handling of the biological specimen includes prevention of contamination and cross-contamination, prevention from exposure foreign particles and pathogens.
3. Which diseases can be diagnosed using biological specimens?
The biological specimens are used to diagnose many diseases such as cancer, HIV, auto immune disorders, various infections, etc.
4. How do biological specimens contribute to public health research?
The biological specimens contribute to public health research by helping the scientists and researchers understand the diseases patterns, their prevention and treatment and how their vaccines can be developed and the risk factor associated with it.