- The production of medical devices in Thailand was estimated around 4.9 million U.S. dollars in 2022.
- The total market size of medical devices forecast is approximately 3.4 billion U.S. dollars by 2027.
- Medical devices exported from Thailand are typically single-use devices such as medical rubber gloves, catheters and medical tubing, syringes and hypodermic needles, and bandages and dressings.
Thai Regulatory Authority For Medical Devices
The Thai Food and Drug Administration (FDA) operates under the Ministry of Public Health and is the primary government agency responsible for ensuring the safety, quality, and efficacy of healthcare products, including medical devices. The Medical Device Control Division (MDCD) of the Thai FDA oversees the regulations and registration processes of medical devices.
Classification of Medical Devices In Thailand
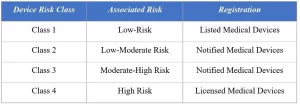
Class I: Low-risk medical devices that are simple in design and pose minimal risk to patients or users. Examples include basic surgical instruments, bandages, and examination gloves.
Class II: Moderate-risk medical devices that are more complex than Class I devices and may require specific controls. Examples include X-ray machines, electrocardiographs (ECGs), and infusion pumps.
Class III: High-risk medical devices that are complex and could pose significant risks to patients if they malfunction. These devices often require pre-market approval and comprehensive regulatory oversight. Examples include implantable pacemakers, heart valves, and certain life-supporting devices.
Class IV: Very high-risk medical devices, usually those that are new or innovative and have not been previously used in Thailand. These devices undergo the most rigorous evaluation and are subject to strict regulatory scrutiny. Examples include certain advanced surgical implants and cutting-edge medical technologies.
Medical Device Labeling Requirements In Thailand
For Class I Medical Devices, labeling requirements include:
- Name and address of the manufacturer or authorized representative.
- Device model or catalog number.
- Intended use of the device.
- Date of manufacture or lot/batch number.
- Clear instructions for use.
- Any warnings or precautions.
- Information on storage conditions and shelf life if applicable.
In addition to the requirements for Class I devices, Class II, III, and IV devices, typically have more comprehensive labeling requirements:
- Detailed technical specifications and performance characteristics.
- Instructions for installation, operation, and maintenance.
- Description of the device’s principles of operation.
- Information on contraindications and side effects, if applicable.
- Precautions for use and potential risks.
- Information on sterilization or cleaning procedures, if applicable.
- Information on compatible accessories or components, if relevant.
Language Requirements: Labels should be in Thai or have a Thai translation included.
Symbols and Icons: The use of symbols and icons on medical device labels is acceptable if they are widely recognized and understood by healthcare professionals and end-users. However, the Thai FDA may require the inclusion of explanatory text for certain symbols.
Packaging Requirements: The packaging of medical devices should also contain important information, including the name and address of the manufacturer or authorized representative, device model or catalog number, and essential instructions for use. Additionally, the packaging should be designed to protect the device during storage and transportation.
Clinical Trial Requirements In Thailand For Medical Device:
For higher-risk medical devices, pre-clinical and clinical testing is required and the key requirements for conducting clinical trials in Thailand include:
Ethics Approval: Before starting any clinical trial, researchers must obtain approval from an ethics committee or institutional review board (IRB) in Thailand. The ethics committee evaluates the study protocol to ensure that it meets ethical standards and safeguards the rights, safety, and well-being of trial participants.
Clinical Trial Authorization: Sponsors or researchers must submit a clinical trial application to the Thai FDA for authorization before commencing the trial. The application should include detailed information about the investigational product, study protocol, safety data, and other relevant documentation.
Informed Consent: Informed consent must be obtained from each participant before their enrolment in the clinical trial. Participants must be provided with comprehensive information about the study’s purpose, procedures, potential risks, and benefits, and they must voluntarily agree to participate.
Good Clinical Practice (GCP): Clinical trials in Thailand must adhere to international standards of Good Clinical Practice. GCP guidelines ensure the quality, integrity, and ethical conduct of the trial and are designed to protect the rights and well-being of trial participants.
Safety Reporting: Researchers and sponsors are required to report any serious adverse events (SAEs) or unexpected adverse reactions that occur during the trial promptly. These reports are submitted to the Thai FDA and the ethics committee.
Product Testing For Medical Devices
Performance Testing: Medical devices must undergo performance testing to ensure they meet the required technical specifications and performance characteristics. This testing assesses factors like accuracy, sensitivity, precision, and durability.
Biocompatibility Testing: Biocompatibility testing is essential to determine how a device interacts with the human body and whether it causes any adverse reactions or complications.
Electrical Safety and Electromagnetic Compatibility (EMC) Testing: Devices that involve electrical components or emit electromagnetic radiation must undergo testing to ensure they meet safety standards and do not interfere with other medical devices or electronic equipment.
Sterilization Validation: Medical devices that are intended to be sterile must undergo validation testing to ensure that the sterilization process effectively eliminates all microorganisms and maintains sterility until the point of use.
Usability and Human Factors Testing: Usability testing evaluates how easy the device is to use by its intended users and assesses the risk of human error during operation.
Device Conformation Assessment
Documentation Review: Manufacturers or their authorized representatives in Thailand are required to submit the necessary documentation to the Thai FDA for review. The documentation may include technical specifications, test reports, clinical data (if applicable), and information about the manufacturing process.
Conformity Assessment Body (CAB) Review: In some cases, depending on the risk classification and type of medical device, the Thai FDA may involve a CAB in the evaluation process. The CAB is an independent third-party organization responsible for assessing the device’s conformity with the applicable regulations and standards.
Quality Management System (QMS) Review: Manufacturers are expected to have a robust Quality Management System in place to ensure consistent product quality and compliance with relevant standards. The Thai FDA may review the QMS as part of the conformity assessment.
Medical Devices Registration Process In Thailand
Preparation of Documents: Manufacturers or their authorized local representatives are required to prepare and submit a set of documentation for the medical device. The specific documents needed may vary depending on the device’s classification but typically include:
- Application form for medical device registration.
- Device technical specifications and detailed description.
- Information on the manufacturing process and quality control.
- Product labeling and packaging details.
- Certificate of Free Sale (CFS) or Certificate of Origin (COO) from the country of origin.
- Summary of pre-clinical and clinical testing data (if applicable).
- Conformity assessment documentation.
Submit the Application: Once all the necessary documentation is prepared, the application for medical device registration is submitted to the Thai FDA. The application should be completed accurately and include all required information to avoid delays in processing.
Review and Evaluation: The Thai FDA reviews the submitted documents to assess the device’s safety, quality, and conformity with applicable regulations and standards. This review process may involve the involvement of a Conformity Assessment Body (CAB) for higher-risk devices.

Approval and Registration: If the medical device is found to meet the required standards and safety criteria, the Thai FDA issues an approval letter, and the device is registered for market entry in Thailand.
Validity and Renewal: A license for medical devices is issued for a period of 5 years. Renewals are less expensive than initial registration applications for Class 1 Listings. However, for Class 2-4 devices the registration renewal is the same as the initial registration fee. As the expiry date approaches, pharmaceutical companies need to apply for the renewal of the product’s registration to continue marketing the product legally in the country. The renewal process involves updating the product’s documentation and ensuring that it remains compliant with current regulations.
Products approved in the EU/US help in fast-tracking the registration in Thailand
The International Recognition status of the medical device is leveraged by The Thai FDA. Prior approval from international agencies such as including the European Medicines Agency (EMA) and the United States Food and Drug Administration (FDA) is sometimes acknowledged by the Thai FDA and considers it as a reference during the review process in Thailand which eases the registration process by:
Reduced Documentation Requirements: If a medical device is approved or cleared in the EU or US, some of the technical documentation submitted to these regulatory agencies could be used as part of the registration application in Thailand. This may reduce the burden of duplicate documentation and streamline the process.
Expedited Review: In certain cases, the Thai FDA may expedite the review process for medical devices with approvals or clearances from reputable international agencies. However, the exact criteria for expedited review are subject to the Thai FDA’s discretion and may vary depending on the device’s risk classification.
Import and Distribution Requirements
Import License: Companies or distributors intending to import medical devices into Thailand must obtain an Import License from the Thai FDA. The application process typically involves submitting relevant documentation, including the device’s technical specifications, proof of conformity assessment, and device details.
Importation Process: Medical devices must be imported through designated ports of entry in Thailand. Importers should follow the customs clearance process and adhere to any importation-related requirements set by the Thai Customs Department.
Good Distribution Practice (GDP): Companies involved in the distribution of medical devices must comply with Good Distribution Practice guidelines to.
- Quality Management – clearly defined in procedures and systematically reviewed and implemented.
- Personnel – There must be sufficient competent personnel to carry out the responsibilities.
- Premises and Equipment – There must be suitable, clean premises and equipment to ensure the proper storage, transportation, and handling of the devices to maintain their quality and integrity.
Documentation – Documentation should be clear and concise and there should not be any room for errors and permits the tracking of relevant operations during the distribution process.
Post-Market Surveillance: Importers and distributors should actively monitor the safety and performance of the medical devices in the market and report any adverse events or issues to the Thai FDA.
Pharmacovigilance: Pharmaceutical companies are required to have a robust pharmacovigilance system in place to promptly detect and address any potential safety issues. The Medical Device Control Division of the Thai FDA has laid down a well-structured post-market subdivision that oversees the PMS compliance of medical devices which includes:
- Report any device defects or Adverse Events (AE) happening to end-user to the Thai FDA
- Field Safety Corrective Actions (FSCA) taken in order to eliminate the device defect or AE risks shall be reported to the Thai FDA.
- Evaluation of the cause of the device defect or AE and FSCAs requires submission of the following information.
- Description of the device defect or AE, and FSCA
- The place where a device defect or AE occurred
- Persons affected by the incident (only for AE)
- Health hazard evaluation report or any related documents, together with the initial report (only for the FSCA)
NOTE: Reporting of any adverse events or defects shall be reported to Thai FDA within 48hrs to 30 days depending on the severity of the adverse event.
Variations: Variations in the context of post-marketing activities refer to any changes or modifications made to the product’s registered details or labeling. These changes may include updates to the product’s composition, manufacturing process, dosage form, strength, packaging, or indications for use. Depending on the nature and impact of the variation, pharmaceutical companies may need to submit applications for approval to the Thai Food and Drug Administration (FDA) to update the product’s registration accordingly.
Audits: Pharmaceutical companies may be subject to regulatory audits by the Thai FDA to assess their compliance with relevant laws, regulations, and good manufacturing practices (GMP). These audits can occur at any time during the product’s lifecycle and are part of the authorities’ efforts to ensure the quality and safety of pharmaceutical products in the market.
Medical Device Registration Pathways In Thailand

Medical devices manufactured or imported into Thailand may serve either commercial or non-commercial purposes. For medical devices manufactured with in Thailand or imported into Thailand for commercial purposes, there are three distinct review pathways available to secure approvals from the Thai FDA:
- Full evaluation
- Concise pathway
- Reliance program
Medical Device Registration via Full Evaluation Route
The manufacture or importation of medical devices in Thailand for commercial use necessitates the acquisition of the following:
- Establishment Licensing for Manufacturing or Importing
- Product Registration for Manufacturing or Importing
If an applicant is uncertain whether their product qualifies as a medical device, they have the option to submit a Product Classification Application to the Agency for clarification. This helps ensure accurate categorization and adherence to regulatory requirements.
Establishment Licensing
Anyone intending to engage in the manufacturing or importation of medical devices must complete the establishment registration process with the Thai FDA. This step is a fundamental requirement to ensure compliance with regulatory standards.
Product Registration
Once the Establishment license has been obtained as a prerequisite, the manufacturer or importer must initiate the product registration process. The initial step in the medical device registration process involves classifying the medical device into a specific risk category. Depending on this classification, the medical device will either be listed for low-risk devices or notified in the case of low-moderate and moderate-high risk devices.
Class 4 high-risk devices are subject to rigorous scrutiny and must receive licensing from the Thai FDA. It’s important to note that the documentation requirements and review timelines differ for Listed, Notified, and Licensed Medical devices, corresponding to their respective risk classifications.
The applicant is required to submit the application along with the requisite documentation and the applicable review fees. The specific fees are determined based on whether the device falls into the listed, notified, or licensed category, each having its own corresponding fee structure.
Documentation requirements for Listed, Notified and Licensed Medical Devices

When seeking to renew the certificate of a Notified or licensed medical device, the registration holder must submit an application for renewal prior to the certificate’s expiration date. This application should be accompanied by the certificate of the Listed, Notified, or licensed medical device, as well as any necessary information, documentation, or supporting evidence as specified in the application form. Additionally, the renewal fee should be included with the submission. This ensures the seamless continuation of certification for the medical device.
In the event of the certificate of a Listed, Notified, or licensed medical device being lost, damaged, or destroyed, the registrant is obligated to submit an application for a replacement certificate of the licensed medical device to the licensor within fifteen days from the moment the loss, destruction, or damage is acknowledged. Additionally, it is mandatory to return the damaged certificate of the licensed medical device, or if applicable, provide a police report concerning the loss or destruction of the said certificate, to the licensor. This process ensures the timely issuance of a replacement certificate.
The Thai FDA allows for modifications to be made to the Notified or licensed medical devices. These amendments may pertain to either the establishment license or the product registration. However, when it comes to changes in the establishment registrant’s name and/or alterations to the name or address of the facility engaged in the manufacturing or importation of medical devices, the registrant can only make amendments to the certificate after the necessary adjustments have been approved in the medical device manufacturing or importing establishment license.
When seeking amendments to the certificate of the licensed medical device, the registrant is required to submit an application for the modification of the approved items within the certificate. This application should be accompanied by the pertinent information, documentation, or evidence related to the items slated for amendment. Additionally, other required documentation or evidence as specified in the “Application for Amendment to Approved Items in the Certificate of Licensed Medical Device” form should also be included in the submission. This comprehensive approach ensures that any changes are processed efficiently and accurately.
Thailand’s medical device regulations is stringent and complex, to know more about the intricate details, reach out to Artixio for expert assistance.
References:
- https://clinregs.niaid.nih.gov/country/thailand
- https://permitfortraveler.fda.moph.go.th/nct_permit_main/
- https://nsp.mohw.org.tw/dl-34-960f0775f3234877a9cde3b445548c62.html





