Cosmetics are huge in Saudi Arabia right now, and the government is paying close attention. SFDA, which oversees beauty products, food, and drugs, makes sure every cosmetic product is registered before it hits the shelves. No shortcuts here—even a single lipstick needs to be listed with them.
The whole thing might look complicated at first. There’s more than one portal, lots of paperwork, and a few steps that can trip you up if you miss them. But once you know the flow, it starts to make sense. That’s what this guide is here for—you’ll get a clear overview of SFDA cosmetics regulations in Saudi Arabia and what they mean for your products.
Who Regulates Cosmetics in Saudi Arabia?
SFDA (Saudi Food and Drug Authority) is the agency that regulates cosmetics in Saudi Arabia. They’re the same body that keeps an eye on food, medicine, and medical devices too—not just beauty products. Within SFDA, there’s a dedicated Cosmetic Products Department that deals specifically with:
- Reviewing cosmetic ingredients and formulations.
- Checking product safety before and after market launch.
- Setting rules for labeling, packaging, and advertising.
Also Read: Cosmetics Regulatory Compliance Services
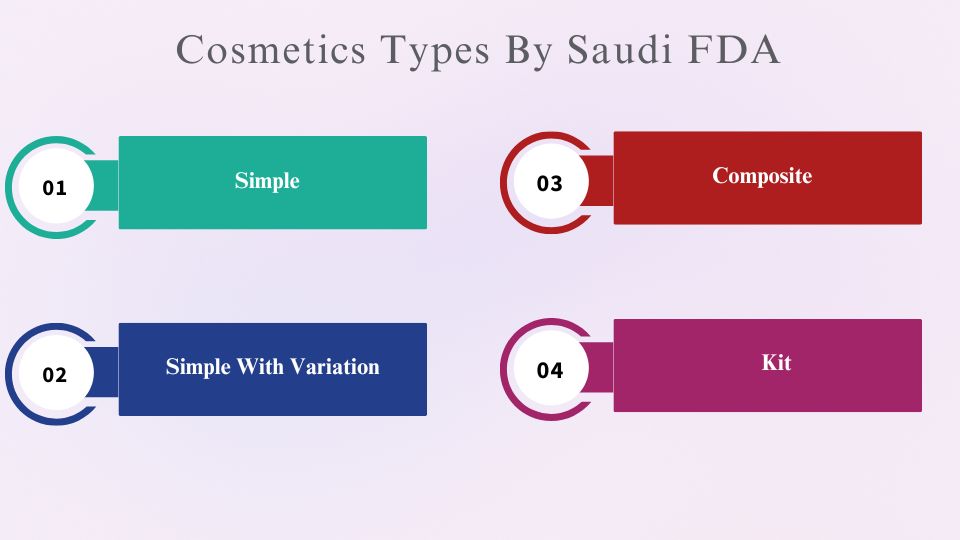
Types Of Cosmetics According To SFDA:
The cosmetic in Saudi Arabia is classified by the SFDA into the following categories:
- Simple:
- Simple cosmetics involve single component products.
- It has one product package, with one information leaflet and belongs to one category.
- Simple Variation:
- It is a simple cosmetic product with a change in fragrance or shade such as lipsticks.
- This cosmetic product may have changed fragrance, shade, strength, etc.
- It has a single product information file belonging to a single product category, just with some variations.
- Composite:
- This category involves a composite product such eye shade palette, lips palette, etc.
- It is basically a set comprised of individual components.
- It has a single information file.
- And each product of the composite product may belong to one or more categories.
- Kit:
- It is a composite product containing several individual single products.
- Each product will have its individual information file.
- Saudi FDA (SFDA) Regulation of Cosmetics in Saudi Arabia
Key requirements Of SFDA For Cosmetics:
The following are the requirements for cosmetics in Saudi Arabia:
- Before you even upload a single document on SFDA’s eCosma / GHAD portal, make sure your product checks the main compliance boxes:
- No prohibited ingredients – Certain substances are completely banned. (SFDA publishes a prohibited list.)
- Ingredient safety – Even approved preservatives or colorants can only be used within set limits.
- Labeling rules – Labels must be clear, accurate, and in both Arabic and English. No exaggerated or false claims.
- Product Information File (PIF) – You’ll need to maintain a detailed file with formulation, safety data, and testing results for SFDA inspection.
- Advertisement control – Marketing must stick to facts—“medicinal claims” can lead to penalties.
👉 In short: if your product isn’t safe, properly labeled, or backed by a PIF, SFDA can block it before it reaches shelves.
Steps to Register Cosmetics in Saudi Arabia
Here are the quick steps for the registration process of the cosmetic products in Saudi Arabia by SFDA:
1. Confirm the category
Make sure your product is considered a cosmetic under SFDA rules (not a drug or medical device).
2. Create a GHAD account
Your Saudi legal entity or importer must register in SFDA’s GHAD system.
3. Prepare your documents
- Full ingredient list (INCI + %)
- Draft labels (Arabic + English)
- Product safety file (PIF) with testing + GMP proof
- Certificates for any restricted ingredients
4. Notify the product in eCosma
Submit product details, upload documents, and get your cosmetic listing.
5. Apply for activity licenses
Depending on your role (importer, distributor, or manufacturer), get the required SFDA license via GHAD.
6. Request Certificate of Conformity (CoC)
Before importing, apply through the FASEH system for shipment clearance.
7. Clear goods at port
Use the FASAH ↔ GHAD platform to complete customs + SFDA checks.
8. Finalize labeling
Ensure packaging follows SFDA/GSO 1943 rules: Arabic & English labels, batch/expiry, importer info, usage/warnings.
9. Monitor after launch
Report serious side effects, keep records updated, and provide the PIF if SFDA requests it.
Lifecycle Of Cosmetic Products In Saudi Arabia: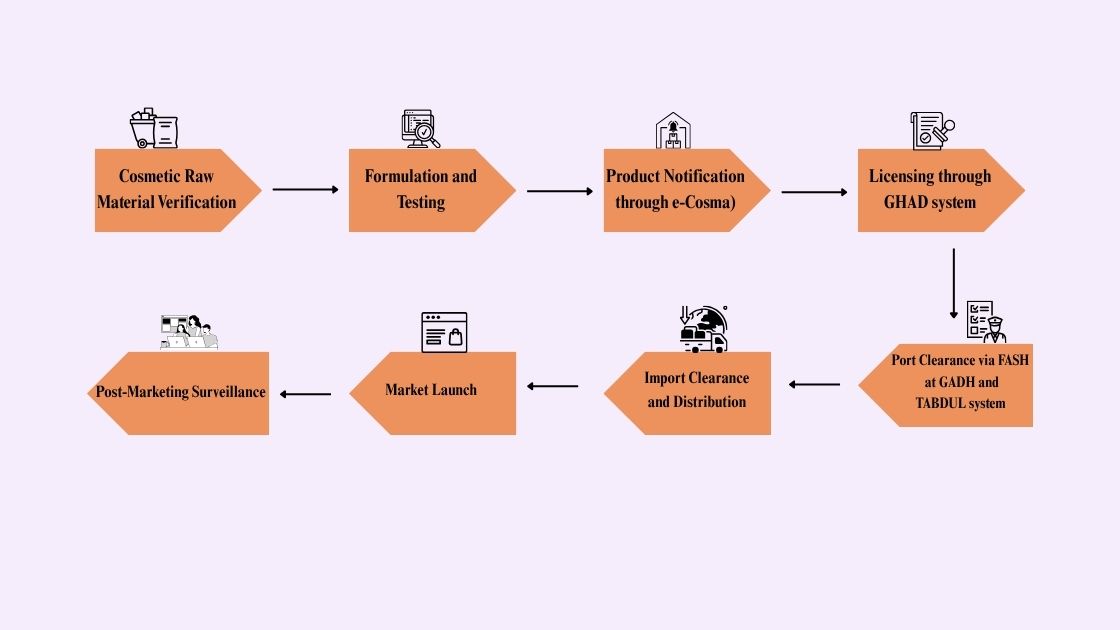
In Saudi Arabia, cosmetics are regulated by the Saudi Food and Drug Authority (SFDA) under the Implementing Regulation of the Cosmetic Law (Royal Decree M/49, 2015).
The first step is checking if your product is actually classified as a cosmetic under SFDA rules. Once that’s confirmed, you can move through the registration process. Here’s the each step of lifecycle most cosmetic products follow in Saudi Arabia:
Cosmetic Raw Material Verification:
This is the first step in the cosmetic manufacturing process.
- All the raw materials used should comply with the SFDA.CO/GSO 1943- Clause 6-2 and its latest updates, if any.
- While selecting the raw materials for cosmetics, the manufacturer should take care that he avoids the ingredients from the prohibited list. To check whether your ingredients fall on the prohibited list, click here.
- All the ingredients used should be approved by the SFDA and should be safe to use.
- If using restricted preservatives in cosmetics, it should be used only under regulated limits.
- All the mandatory raw material certificates should be uploaded to the eCosma portal.
Formulation and Testing:
- The formulation and testing of the cosmetics are regulated under SFDA.CO/GSO 1943 – Annex 3 & Clause 5-2
- It is mandatory for manufacturers to carry out formulation and under GMP standardized facilities that is ISO 22716.
- During the manufacturing process, a technical product file (PIF) should be maintained for SFDA review.
- During manufacturing and post-manufacturing all the necessary tests should be conducted on cosmetic products such as stability studies, shelf life, etc.
Product Notification Through eCosma:
- According to the SFDA.CO/GSO 2528 Notification Guide, all the product related data should be submitted to the eCosma.
- This notification process is meant to add cosmetic products to the records after been approved by the Authority.
- A cosmetic product cannot be imported or traded in the Kingdom of Saudi Arabia unless it is notified in the Unified Electronic System (GHAD), which is an online portal specially designed for the cosmetic manufacturers to notify their product.
- To know more about how to register on GHAD portal, click here.
Some Requirements For Product Notification:
- Labeling:
- A proper image should be provided of the front and back label including the barcode.
- The label should be prepared in compliance with the SFDA standards.
- Ingredients:
- A dossier should be submitted that includes all the ingredients along with their concentration.
- The notifier should confirm that there are no ingredients on the prohibited ingredients list.
- The notifier is solely responsible for the information on the dossier.
- Documents:
Following is the list of documents required for creating GHAD account:
-
- Commercial registration record.
- Copy of Ministry of Investment License.
- Notarized Authorization from the Chamber of Commerce.
Following is the list of documents for notifying a cosmetic product:
- Notarized copy of authorization letter from the manufacturer.
- Image of product label.
- Image of product for publication.
- List of all ingredients with their concentrations and mode of action.
Renewal Of Cosmetic Product Listing:
- The cosmetic product listed by the authority for approval needs to be renewed before its expiry.
- For the renewal process, the manufacturer must apply 90 days before the expiration date.
Deactivation Of Cosmetic Product Notification:
- The notifier has the right to deactivate his cosmetic product from the list anytime he wants.
- For doing so, he needs to login into the eCosma portal and select product services.
- And then choose cosmetics products, then search for your product in the list and deactivate the product.
To know more about the Cosmetic Notification process in Saudi Arabia, click here.
Licensing Through GHAD System:
After the notification process, the applicant needs to apply for license for his intended cosmetic product use in Saudi, such as import, manufacture, sale,etc.
The Implementing Regulation – Article 13 states all the necessary requirements for licensing cosmetics.
Following is the process for licensing by SFDA:
- Register for a GHAD account.
- Select the appropriate licensing type based upon the intended use such as manufacturing, distributing, import, etc.,
- Upload the required documents and pay the fees and finally submit the application.
- The SFDA carries out inspection of the applied license faculty.
- If all parameters are found to be authentic, the applicant is granted the license.
- The GHAD portal is used to update any documents, renew licenses, or any cosmetic related procedure later after the grant of license.
Port Clearance via FASH at GADH TABDUL System:
- According to the SFDA.CO/GSO 1943 – Clause 6-3, on the arrival of cosmetics on port, for import or export, the person in-charge is required to submit clearance request using FASH at GHAD and Fasah Tabadul system.
- This clearance request should be submitted before the cosmetics stock reaches the port using the Fasah Tabadul which is integrated with GHAD.
- The documents such as product details, GHAD license, Fasah Tabadul clearance request, import/export purpose, lab testing certifications should be produced at the port.
- Original clearance documents should be preserved for a period of atleast 5 years, so that they can be reproduced as and when required.
SFDA Cosmetics Import Clearance And Distribution:
- The SFDA.CO/GSO 1943 – Clause 7; GSDP Guide states all the requirements for the import clearance and distribution of cosmetics.
- Now, all the process of shipment is automated through the GHAD portal, hence speeding up the import clearance and distribution.
The following are some of the essential requirements for import clearance and distribution:
- GHAD Notification: It is essential to notify the product through GHAD before the import.
- Certificate of Conformity (Coc): This CoC is issued to only produced that have been notified to GHAD. It is a mandatory document and is issued through Fasah system.
- Importer Account & Licensed Warehouse: The importer must have an SFDA GHAD account, and the warehouse should be licensed.
- Compliance with GSO 1943/2016: The cosmetic shipments should comply with the GSO 1943/2016 cosmetic technical regulations.
- Good Storage & Distribution Practices: The shipments should be stored in the warehouse in compliance with all the Good Storage & Distribution Practices.
- Documentation: Documents such as test reports, ingredient lists, safety data, label copies, etc. should be produced at the time of shipment.
Cosmetic Products Market Launch in Saudi Arabia:
- SFDA.CO/GSO 1943 – Clause 5 regulates the Market Launch.
- Once the produced is successfully notified and authentic facility license is granted, the product can enter the Saudi market.
- Manufacturing should ensure proper labelling before its market entry.
- The label should be prepared in English as well as Arabic, with correct ingredient list and barcode mentioned.
- It is mandatory that the product owns a CoC, and its distribution warehouse complies with Good Storage and Distribution Practices.
Post-Marketing Surveillance:
- Under the SFDA.CO/GSO 1943 – Clause 8 & 9, post–marketing surveillance activities are regulated.
- Under the PMS, undesirable effects, consumer complaints, serious events, etc. are reported.
- Upon major serious effect, the product should be recalled within 10 days from the market.
- And serious reactions and manufacturing defects should be reported to the SFDA within 20 days.
Fees Associated With Cosmetic Products:
In Saudi Arabia, there is no fees associated with the cosmetic product registration; however, the licensing of companies and warehouses involves certain fees.
Conclusion:
Thus, we get to understand that the Saudi FDA imposes certain requirements for the marketing and importation of cosmetics. However, this is a notification process and does not require any approval. But the manufacturers and importers should ensure complete compliance of the SFDA regulations considering it as their sole responsibility. If the manufacturers or importers fail to do, the cosmetic may be banned or recalled upon discovery of non-compliance during sudden inspections.
Therefore, to navigate through the complexities of regulatory compliance with Artixio! Contact us today for expert guidance tailored to your needs.
FAQs:
How long does the Saudi FDA take for product approval?
The Saudi FDA takes 4-6 weeks for product approval depending upon the type of product.
What is a Product Information File (PIF)?
A PIF is a cosmetic dossier file required at the time of SFDA inspection. The PIF includes all the information regarding the formulation, manufacturing, labeling, etc.
What are the consequences of non-compliance with the SFDA regulations?
Consequences such as product recalls, suspension, penalties, etc. are faced by the manufacturers upon non-compliance with the Saudi FDA.
Can the same cosmetic product with different shades or fragrances be submitted under one cosmetic notification?
Yes, under the “Single with Variation” category of e-Cosma, the same cosmetic product with different shades or fragrances can be submitted.