Biological Products Compliance Support in India
Expert biologics regulatory consultant in India offering end-to-end services for approvals, documentation, and lifecycle management support.
Artixio is a regulatory services company in India providing clients a complete support through their pharmaceutical commercialization journey. We have wide experience in providing efficient strategies for product registration to maximize commercial potential by reducing time to market. Our team has decades of experience in successfully launching and commercializing life-sciences products.
India’s Biological Market
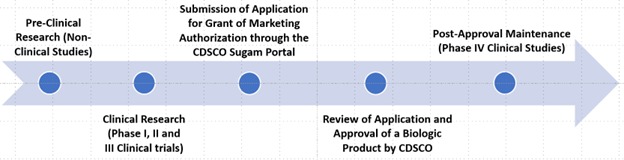
Approval Process for a Biologic Product in India
Approval Process of a biologic product in India involves following steps:
Biologicals Compliance Consulting Services in India | CDSCO Approval
- Product strategy, cost analysis and risk assessment
- Intellectual Property landscape
- Regulatory intelligence and Health Authority insights
- Advisory, Scientific writing and regional support
- Project Management
- Document Management and Publishing
- Compliance driven labeling content
- Data driven decisions right from product development, product launch to post marketing support
- Product branding
Integrated Regulatory Services for Seamless Market Entry in India
Why Choose Artixio for Biological Compliance Services in India?







FAQs
What are the categories for approval of a biological product in India?
- Drug approved in another country/Salt of an Approved drug
- Investigational New Drug
- Approved New Drug
- New Dosage form
- New indication
- New route of administration
What are the different forms available for biological product permission to market, its import and to conduct clinical trials in India?
What are the fees required to be paid for a biological product approval in India?
| Sr. No. | Purpose | Fees Paid |
|---|---|---|
| 1 | Marketing Authorization (Form 44) | Rs 50,000 and Rs 15,000 for regularization of permission |
| 2 | Application for License to import drugs for examination, test or analysis (Form 12) | Rs 100 for each product and Rs 50 for each subsequent product |
| 3 | Subsequent approval of Marketing Authorization (Form 44) | Rs 15,000 |
| 4 | Clinical Trial Phase I (Form 44) | Rs 50,000 |
| 5 | Clinical Trial Phase II (Form 44) | Rs 25,000 |
| 6 | Clinical Trial Phase III (Form 44) | Rs 25,000 |
| 7 | Registration of site (Form 40) | 1,500 USD for each site |
| 8 | Registration of product | 1,000 USD for each product |
| 9 | Import License (Form 8) | Rs 1,000 for each product and Rs 100 for each subsequent product |
How is your regulatory strategy supporting the product evaluation for successful commercialization?
- Therapeutic area, Intellectual property, and competitor’s landscape
- Clinical development roadmap
- Regional Regulatory intelligence and Insights based on the health authority interactions
- Product strategy
- Product cost analysis
- Risk assessment
Still Have Questions ?
Regulatory Expertise Across Multiple Countries












Biologics Product and Market Strategy
Our insights driven Product Roadmap covers,
Competitive
Landscape
Approved Claims
Product Key
Differentiators
Product Branding and Launch Strategy
- Product and Brand Identify
- Branding Guidelines
- Brand Design and Communication Strategy
- Packaging and Artwork Graphic Design
- Marketing Collateral Design and Development
- Merchandize Design
- Digital Content and Design
Communication and Digital
- Biologics Product Label Review
- Biologics Ingredients and Product Compliance Review
- Artwork and Promotional Material Revien
- Product Information File Review (PIF)
- Notification
- Registration with Health Authority
Related India Services
Blogs

CDSCO Simplifies Subsequent Importer
India’s regulatory authority, the Central Drugs Standard Control Organization (CDSCO), has published an important...

CDSCO Medical Device Approval
Central Drug Standard Control Organization (CDSCO) is the regulatory authority that regulates Medical Devices...

IVD Medical Devices Registration
IVDs in India are regulated as drugs under Section 3(b)(i) and 3(b)(iv) of the...





















