Medical Device Regulatory Affairs Services in Indonesia
Artixio helps MedTech companies in Indonesia with device registration support, MoH submissions, and end-to-end regulatory compliance support.
Medical Devices Regulatory Affairs in Indonesia
Artixio offers extensive medical device regulatory services to global manufacturers, facilitating smooth navigation of Indonesia’s MDA regulations. Strategic medical device regulatory affairs consulting in Indonesia, aligned with BPOM regulations and local compliance requirements. We manage device registration, licensing, and approval processes end to end.
Medical devices sold in Indonesia are regulated by the National Agency of Drug and Food Control (NADFC) which is responsible for the import, manufacture, export and supply of medical devices in Indonesia to safeguard public health and safety.
Key Regulations for Medical Devices in Indonesia
Classification of Medical Devices in Indonesia
Registration Process of Medical Devices in Indonesia

Documents Required for Registration of Medical Devices in Indonesia
- Executive Summary
- Device Description
- Design Verification & Validation
- Clinical Evidence
- Device Labelling
- Risk Analysis
- Manufacturer Information
Artixio’s Medical Devices Registration Services in Indonesia
Indonesia Medical Device and IVD Regulatory Approval, from strategic planning and registration to post-market compliance, Artixio provides high-quality regulatory affairs services to support your business needs.
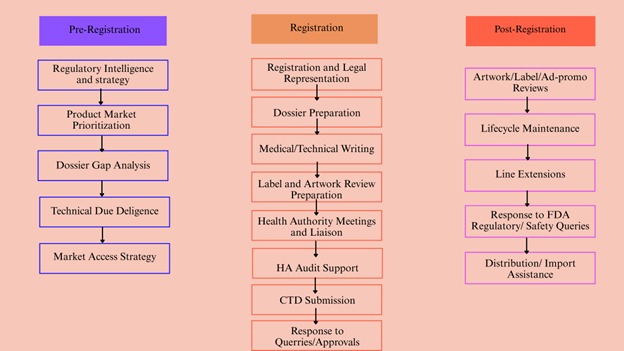
Professional Regulatory Consulting Services in Indonesia
Why Artixio For Indonesia Medical Device Registration and Approval?












FAQs
What is the validity of the medical device license in Indonesia?
Who can Import Medical Devices?
When should the Product License holder apply for a renewal of Product license?
How to Submit the New application for the Product License of Medical Devices?
What is a Production Certificate and who is Producer?
Related Indonesia Services
Industries We Serve in the Indonesia
Regulatory Expertise Across
Multiple Countries












Blogs

Nutraceuticals NADFC Regulations in
The nutraceutical market in Indonesia is expanding, with projections indicating significant growth over the...

Medical Devices Regulations &
Although the manufacturing sector for medical devices in Indonesia is constantly growing, Indonesia is...

NADFC (BPOM) Regulations of
Indonesia’s large population, coupled with a relatively high birth rate, continues to fuel demand...






















