- Home
- >
- Malaysia
- >
- Medtech
Medical Device Regulatory Affairs Services in Malaysia
Regulatory Affairs Services for Medical Devices in Malaysia
Artixio offers extensive Malaysia Medical Device regulatory services to global manufacturers, facilitating smooth navigation of Malaysia’s MDA regulations.
Services

Post-Market Surveillance
Key Regulations for Medical Devices in Malaysia
Classification of Medical devices In Malaysia
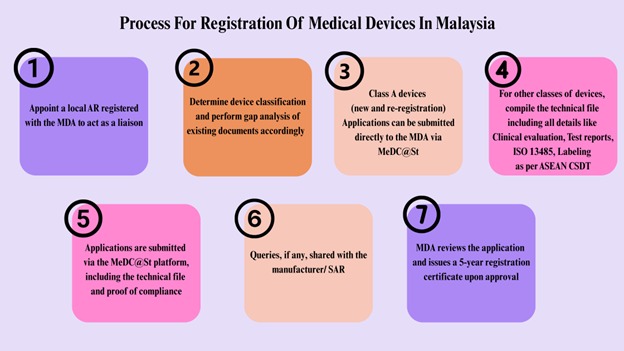
Process for Registration of Medical Devices in Malaysia
Required Documents for Medical Device Registration in Malaysia
- Executive Summary
- Device Description
- Design Verification & Validation
- Clinical Evidence
- Device Labelling
- Risk Analysis
- Manufacturer Information
Artixio’s Medical Device Registration Services in Malaysia
Our Medical Device Regulatory and Market Access experts based in Malaysia, coupled with the Global Regulatory Projects team, have helped over a hundred pharmaceutical, medical devices, diagnostics, cosmetics, nutraceuticals, supplements, and veterinary products successfully register and maintain compliance in Malaysia. Combined with Artixio’s intelligence-based services platform, Dvarka, innovators and manufacturers from around the world have benefited by seamlessly managing the regulatory strategy across the product development and commercialization value chain.
From planning your regulatory pathway to handling approvals and post-market requirements, Artixio helps you manage every step of medical device compliance in Malaysia—efficiently and with confidence.
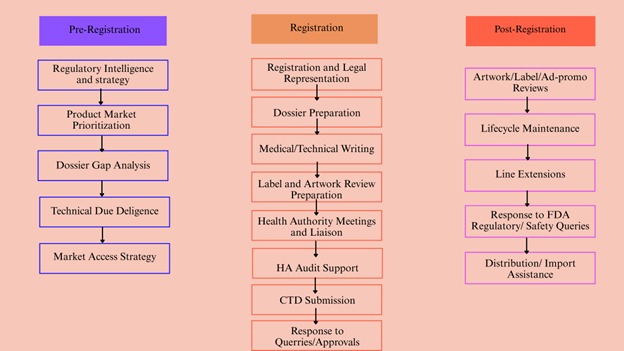
Why Choose Artixio for Medical Device Approval in Malaysia












Market Entry Regulatory Advisory Services – Malaysia
FAQs
If we have created MeDC@St account. Do we need to apply for establishment license, medical device registration or both?
How do we notify MDA in terms of changes (E.g. change manufacturing site from one country to another country etc.)?
Does the authorized representative (AR) require local staff working in the Malaysia office? If our office staff is handling everything from other countries, is this acceptable?
How to apply for re-registration?
Does AR info and registration number need to be put on primary packaging or secondary or both
Related Malaysia Services
Regulatory Expertise Across
Multiple Countries












Blogs

Registration of Biologics in
Biologics have emerged as a significant treatment for various diseases in Malaysia. With their...

Pharmaceutical Regulations and Registration
Before placing a pharmaceutical product on the Malaysian market, it’s important to understand how...

NPRA Health Supplements Regulations
In Malaysia, it’s not unusual to find health supplements being sold online without proper...




























