- Home
- >
- Mexico
- >
- Medtech
Medical Device Regulatory Services in Mexico
Your regulatory partner for Medical devices regulatory affairs services in Mexico, from concept to market. End-to-end regulatory support for medical device registration, compliance, and approvals in Mexico with Artixio’s expert consulting team.
Medical Device Regulatory Affairs Consultant in Mexico
Artixio provides seamless regulatory services for commercialization of your medical device in the Mexican market. Our experienced local team in Mexico helps you navigate through the stringent guidelines of COFEPRIS and achieve timely compliance. We aim at helping our clients right from product conceptualization until product surveillance.
Key Regulations for Medical Devices in Mexico
Classification Of Medical Devices in Mexico
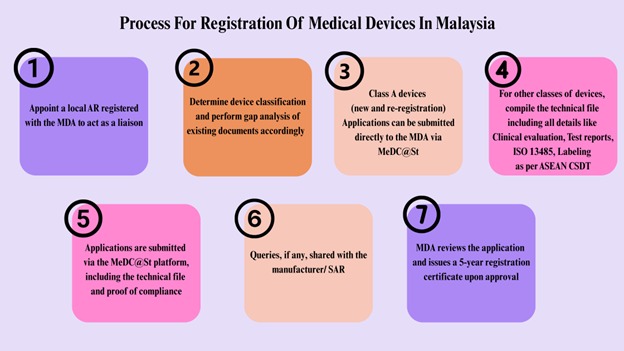
Process For Registration Of Medical Devices In Mexico
Documents Required For Medical Device Registration In Mexico
- Application Form
- Description
- Power of Attorney
- Labeling Information
- Certificate of Free Sale (CFS)
- Clinical Data (for Class II & III)
- Good Manufacturing Practices (GMP) Certificate
- COFEPRIS registration fee receipt.
Artixio’s Medical Devices Registration Services in Mexico
Our Regulatory and Market Access experts based in Mexico, coupled with the Global Regulatory Projects team, have assisted over a hundred pharmaceutical, medical devices, diagnostics, cosmetics, nutraceuticals, supplements, and veterinary products successfully register and maintain compliance with COFEPRIS in Mexico. Coupled with the Artixio’s intelligence-based services platform, Dvarka, innovators and manufacturers around the globe have benefited by seamlessly managing the regulatory strategy for the product development and commercialization value chain.
Mexico COFEPRIS medical device regulations, from strategic planning and registration to post-market compliance, Artixio provides high-quality efficient regulatory affairs services to support your business in Mexico.
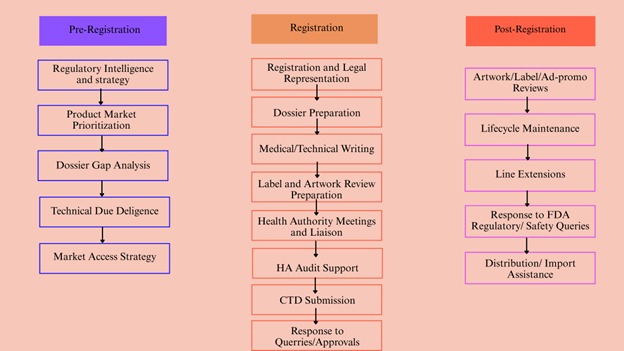
Regulatory & Registration Services in Mexico
Why Choose Artixio for COFEPRIS Medical Device Compliance in Mexico








FAQs
Is it necessary to appoint a local representative in Mexico for medical device commercialization?
Are clinical trials necessary for all medical device classes?
What are the labeling requirements of the medical device in Mexico?
For how long the medical device registration is valid in Mexico and when it should be renewed?
Related Mexico Services
Regulatory Expertise Across
Multiple Countries












Blogs

Mexico COFEPRIS Updates on
Overview of December 2025 Regulatory Changes in Mexico Low risk Medical Device listing, application...

COFEPRIS Simplifies Medical Device
New Regulatory Agreement by COFEPRIS for Simplification of Medical Device Registration Procedures and Shorter...
COFEPRIS Reliance Pathway: Mexico
Modernization of the COFEPRIS Regulatory Framework: Introduction of a Reliance Pathway Diario Oficial de...





















