COFEPRIS (Comisión Federal para la Protección contra Riesgos Sanitarios) is the regulatory authority responsible for pharmaceutical regulations in Mexico. Any pharmaceutical product that is intended to be sold in Mexican market should be registered with COFEPRIS. Mexico is a major market in LATAM region. Streamlining the regulatory procedures for the registration of the pharmaceutical product with COFEPRIS is essential is achieving a successful long-life product in the Mexican market.
Role Of COFEPRIS In Mexican Pharmaceutical Market:
COFEPRIS (Comisión Federal para la Protección contra Riesgos Sanitarios) serves as the main regulatory authority responsible for governing the safety and quality of the pharmaceutical products in Mexico. COFEPRIS also looks over the post marketing surveillance and GMP compliance of the pharmaceutical product after its registration. The COFEPRIS registration is essential for a pharmaceutical product to gain Mexican market access. It also harmonizes with international bodies to meet its standards, Us streamlining the regulatory procedures in Mexico.
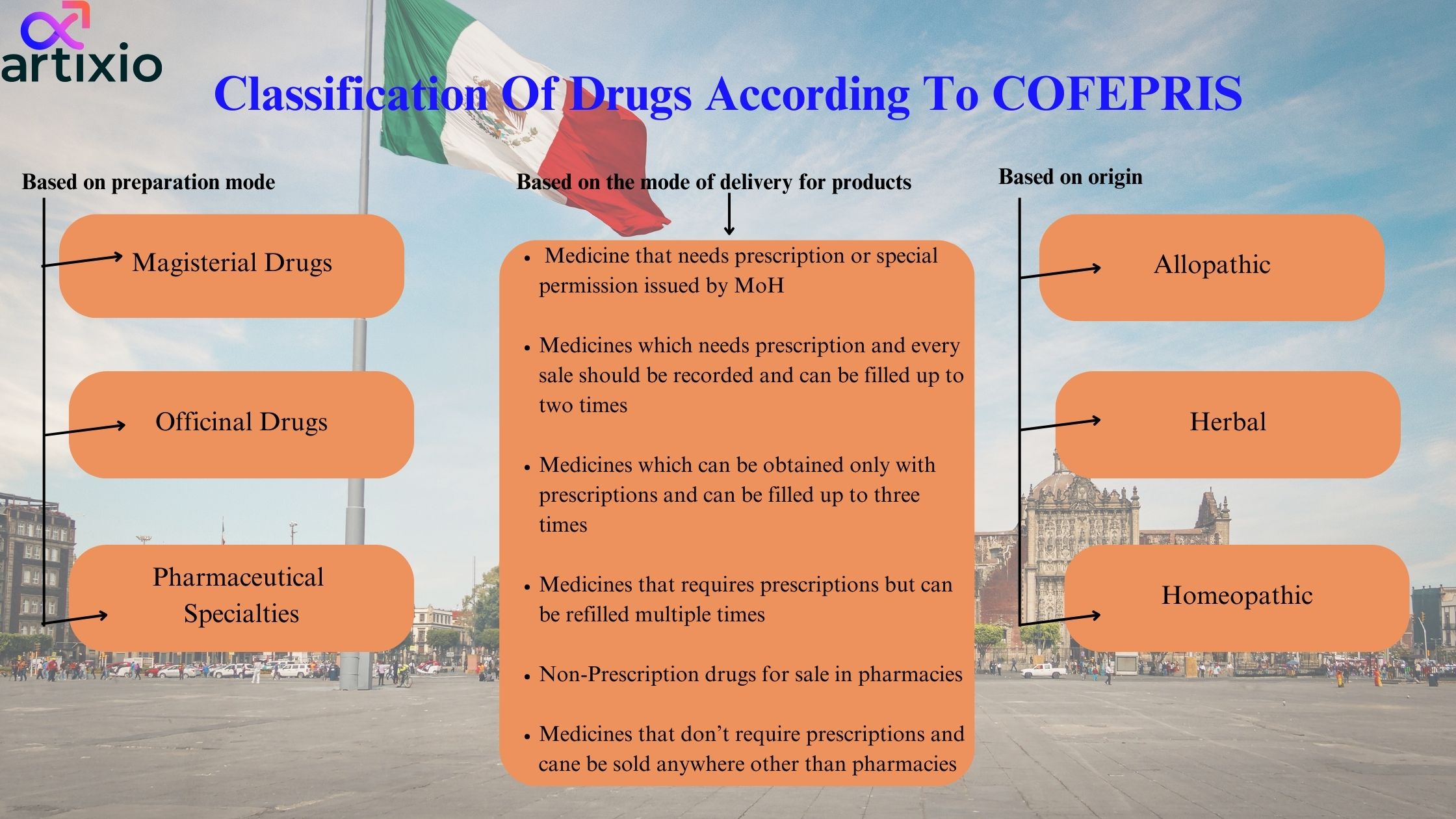
Classification Of Drugs According To COFEPRIS:
COFEPRIS classifies drugs on the basis of preparation mode which categorizes drugs as:
- Magisterial Drugs: These are the ones which are prepared as per doctor’s prescription
- Officinal Drugs: These are as per the Mexican Pharmacopoeia
- Pharmaceutical Specialties: These are the ones prepared with formulas authorized by the Ministry of Health, in collaboration with the pharmaceutical industry
Further it is classified on based of origin:
- Allopathic: Any substance or mixture of substances of natural or synthetic origin that has a therapeutic, preventive or rehabilitative effect, that is presented in a pharmaceutical form ‘and is identified as such by its pharmacological activity, physical, chemical and biological characteristics, and is registered in the Pharmacopoeia of the United Mexican States for allopathic medicines
- Herbal: Products made from plant material or a derivative thereof, whose main ingredient is the aerial or underground part of a plant or extracts and tinctures, as well as juices, resins, fatty and essential oils, presented in pharmaceutical form, whose therapeutic efficacy and security has been scientifically confirmed in national or international literature.
- Homeopathic: Any substance or mixture of substances of natural or synthetic origin that has a therapeutic, preventive or rehabilitative effect and that is prepared in accordance with the manufacturing procedures described in the Homeopathic Pharmacopoeia of the United Mexican States, in those of other countries or other national and international sources of scientific information.
The third classification is based upon the mode of delivery for products; it is further divided into six categories:
- Medicine that needs prescription or special permission issued by MoH
- Medicines which need prescription, and every sale should be recorded and can be filled up to two times
- Medicines which can be obtained only with prescriptions and can be filled up to three times
- Medicines that require prescriptions but can be refilled multiple times
- Non-Prescription drugs for sale in pharmacies
- Medicines that don’t require prescriptions and cane be sold anywhere other than pharmacies.
Pharmaceutical companies planning market entry in Mexico may benefit from experienced regulatory support. Discover how Artixio assists with COFEPRIS pharmaceutical compliance, registrations and ongoing regulatory requirements.
Submission Dossier Preparation:
The layout of the submission dossier depends on the type of product intended to be registered, such as a new molecule, generic, orphan drugs, etc. The format for various product categories is given below:
1. New Molecules:
Module I. Legal/Administrative information
Module II. Quality information
Module III. Preclinical studies
Module IV. Clinical studies
2.Generic drugs:
Module I. Legal/Administrative information
Module II. Quality information
Module III. Bioavailability and/or bioequivalence
3.Vaccines:
Module I. Legal/Administrative information
Module II. Quality information
Module III. Preclinical studies
Module IV. Clinical studies
4.Orphan drugs:
Module I. Legal/Administrative information
Module II. Quality information
Module III. Justification of ‘orphan drug’ status
Module IV. Preclinical studies
Module V. Clinical studies
The process of Registration with COFEPRIS
The process of registration of COFEPRIS begins with the consultation meeting with COFEPRIS followed by the GMP inspection held by COFEPRIS. Both key steps are explained in detailed below:
Consultation Meeting with COFEPRIS New Molecules Committee (NMC):
-
A meeting request is submitted to the committee
-
The NMC reviews the request and share their decision within 60 days
-
NMC schedules meeting with applicant
-
Post meeting with the applicant and reviewing the presentation and documents shared by applicant, NMC issue technical opinion within 20 days
-
Applicant can submit the dossier upon receiving details from NMC
Request GMP inspection from COFEPRIS
-
GMP inspection must be requested to COFEPRIS as Mexico abides to their own CMP practices and standards
-
GMP certificates for other country of origin is exempted
-
GMP certificate is must for all the manufacturing sites
Checklist documents for GMP Inspection
1. Name and general information of the establishment
2. Name of the drug or medicine for which you are requesting verification of GMP
3. Name and full address of the establishment(s) involved in each stage of manufacturing
4. Description of the process that is carried out in each of the establishments involved
5. The manufacturing process for which verification of GMP is requested
6. A list and description of products that are made
7. Name of the legal representative, health officer or person designated by the establishment to attend the diligence
8. Organization charts (general, of the production and quality departments, indicating the reporting lines)
9. Plans of the establishment and production areas
10. Block diagram of the manufacturing process
11. General summary of the quality system including validation and qualification
12. Information from the last two annual review reports, specifically indicating: manufactured lots, rejected lots (indicating reasons), released lots that were subject to investigation, conclusion and actions carried out, number of reprocessed batches, complaints, returns and withdrawal of products from the market, as well as conclusions of the report.
Prepare and Submit registration dossier to third party or directly to COFEPRIS for review and approval.
A Guide To COFEPRIS Modules For Pharmaceutical Registration In Mexico
The COFEPRIS consists of four modules whose structure is shown below:
Module I: Legal/Administrative Information:
-
Application Form
-
Proof of Fee Payment of fee
-
Sanitary authorization (This is applicable for site located outside Mexico, one has to provide license, certificate or other document authorizing the site to the activities related to manufacturing of pharmaceutical products of interest, issued by the competent authority of the country of origin, legalized or apostilled, translated to Spanish by an official translator).
-
Responsible Sanitario notice
Labeling Information Required as per COFEPRIS
-
GMP certificate API manufacturing sites
-
GMP Certificate for Finished product manufacturing sites
-
Certificate of Pharmaceutical Product (This is applicable for drug manufactured outside Mexico)
-
Commercial name of products
-
Intellectual property/Patent Information
-
Information on the waiver for the local manufacturing facility requirement.
Module II: Quality Information
-
API: Manufacturing information, General Information, Quality Control (Literature/Pharmacopeial references. Specifications. Analytical methods. Validation reports. Certificates of analysis (CoAs).
-
Excipients and additives: For new additives, usage safety information, Quality control ((Literature/Pharmacopeial references. Specifications. Analytical methods. Validation reports. Certificates of analysis (CoAs).
-
Finished product: Pharmaceutical development, Formulation/Manufacturing information, in process controls, Quality control, Monographs specifications, Analytical methods certificates of analysis, Stability studies and stability data, Packaging materials (description and capacity)
Module III: Preclinical Studies (as applicable for new molecules, Vaccines and Orphan Drugs)
-
Preclinical studies: Pharmacodynamic studies, Pharmacokinetic studies, Toxicology studies
Module IV: Clinical Studies- Phase I, Phase II, Phase III and Phase IV studies
Registration Process for Foreign Manufacturer:
The process would be different for organizations with prior registration in reference countries or without registration in reference countries
Prior registration in reference countries (USFDA, Health Canada, Swissmedic, EMA, TGA)
- Request meeting with COFEPRIS New Molecule Committee (NMC)
-
Using 3rd party for evaluation of technical files
-
3rd Party issues evaluated report to Manufacturer
-
Submit registration request to COFEPRIS
Common Challenges Encountered In COFEPRIS Registration:
Some of the common challenges faced by the applicants who wish to register their products with COFEPRIS is as follows:
Complex Regulatory Requirements:
The COFEPRIS has a detailed complex regulatory requirement which might sometimes become difficult to follow. This can be overcome by being updated with the latest new updates and hiring a local experienced representative in Mexico.
Language Barrier:
As the complete regulatory procedure in Mexico is in Spanish language it may create a barrier for the foreign people who wish to register their products in Mexico. This problem can be overcome by correctly translating all the requirements.
COFEPRIS Timelines:The review time for COFEPRIS registration might be long due to many registrations handled by COFEPRIS simultaneously. This can be overcome by ensure a correct and authentic registration application to avoid additional non-compliance correction timelines.
High Costs of the Registration Fees:
The complete process of pharmaceutical product registration with COFEPRIS can be very costly for the applicant to afford sometimes, hence the applicant should plan out the budget first and then only register with COFEPRIS.
COFEPRIS Registration Fees:
The COFEPRIS registration fees depends on the type of product, its complexity and the according to the risk associated with it. However, the COFEPRIS registration fees are high therefore one should plan its budget before registering with COFEPRIS and stay updated with the latest changes in the fee structure.
The table below explains the approximate registration fee for various products:
| PRODUCT TYPE | REGISTRATION FEES |
| Generic Drugs | MXN 82,012 approx. |
| New Molecule | MXN 146,642 approx. |
| Class I Medical Devices | MXN 13,000 approx. |
| Class II Medical Devices | MXN 20,000 approx. |
| Class III Medical Devices | MXN 25,000 approx. |
Timelines by COFEPRIS
The response time for New Molecules and Generics is anywhere around 180 days whereas requesting a meeting with COFEPRIS New Molecule committee takes around 60 days and another 20-40 days for receiving New Molecule committee conclusions after meeting.
At Artixio, we have a team of experts who understand the Mexican market and comes with decades of experience working with the authorities in Mexico. Our team has helped numerous pharmaceutical companies complying to COFEPRIS’s regulatory needs. Our experts have expertise in providing end to end solution for Mexican registration requirements:
-
Preparation and submission of dossier, complying to necessary guidelines to ensure a smooth process of submission for pharmaceutical products
-
We have a team of consultants who have experiences handling GMP audits for manufacturing sites updating system as per GMP requirements
-
Artixio team can partner as your authorized legal representative for Mexico helping with health authority (COFEPRIS) communication on a regular basis
-
We have our partners who can also assist you with local clinical and non-Clinical testing of your products at ISO compliant laboratories in Mexico
COFEPRIS Introduces Changes to Drug Registration Process in Mexico
The Mexican health authority, COFEPRIS (Comisión Federal para la Protección contra Riesgos Sanitarios), has a new drug registration process that went into effect in January 2023 in Mexico. The new process is designed to be more efficient and transparent, and it includes a number of changes from the previous process.
Here are some of the key changes to the drug registration process in Mexico:
- The new process is based on the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines. This means that the requirements for drug registration in Mexico are now more aligned with the requirements in other countries.
- The new process includes a pre-submission consultation meeting with COFEPRIS. This meeting allows the applicant to discuss their drug registration dossier with COFEPRIS before submitting it, which can help to expedite the review process.
- The new process includes a single review team for all aspects of drug registration. This means that the applicant only has to deal with one team of reviewers, which can help to streamline the review process.
- The new process has shorter review times. The average review time for a new drug registration dossier is now 12-18 months, compared to 24-36 months under the previous process.
The new drug registration process in Mexico is a positive step towards ensuring that safe and effective drugs are available to Mexican patients more quickly. The process is more efficient and transparent, and it includes a number of changes that are designed to streamline the review process.
Here are some of the key documents that need to be submitted as part of the new drug registration process in Mexico:
- Sanitary Registration Application: This is the main document that is submitted to COFEPRIS. It includes information on the drug’s safety, efficacy, and quality.
- Clinical Trial Report: This document reports the results of the clinical trials that were conducted to support the drug’s safety and efficacy.
- Manufacturing Information: This document provides information on the drug’s manufacturing process.
- Labeling and Packaging Information: This document provides information on the drug’s labeling and packaging.
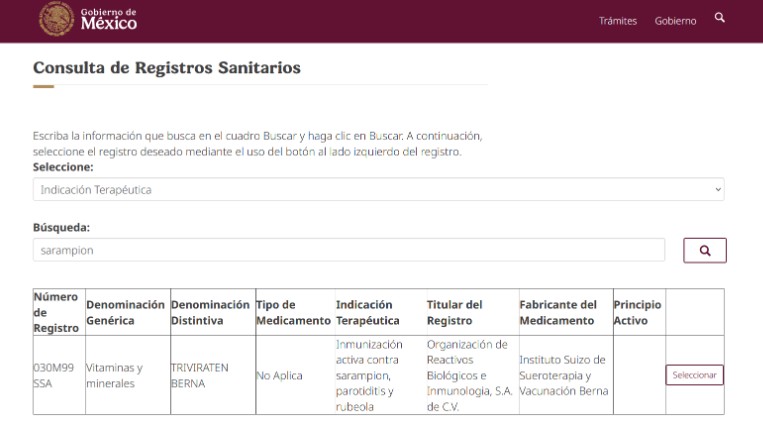
January 2026 Update: Public Access to Pharmaceutical Premarket Authorizations
It is possible to review current premarket authorizations for medication here:
The site can be searched by:
- Premarket authorization
- Generic name
- Distinctive name
- Type of medication
- Therapeutic use
- Name of premarket authorization holder
- Name of medication manufacturer
- Active ingredient
I chose for the example “therapeutic use” and added “Measles”, and got what follows:
If “Seleccionar” is chosen on the site from this example, then the full information is displayed.
Conclusion
The new drug registration process in Mexico is a complex process, but it is designed to ensure that safe and effective drugs are available to Mexican patients. If you are interested in registering a drug in Mexico, you should contact a qualified regulatory affairs consultant to help you through the process.
Our team would be happy to assist you with your requirements for Pharmaceutical Product regulatory affairs in Mexico. Connect with us at info@artixio.com
FAQs:
Why is COFEPRIS important for pharmaceutical registration in Mexico?
COFEPRIS is important as it is the regulatory authority governing in Mexico, which checks and confirms the safety, efficacy and quality of pharmaceutical products and grants approval for marketing of the same.
Can a pharmaceutical product be registered in Mexico without COFEPRIS registration?
No, it is compulsory for a pharmaceutical product to register with COFEPRIS in Mexico.
How can the COFEPRIS registration process be tracked?
The COFEPRIS registration process can be tracked through its online portal.
Can a pharmaceutical product be advertised before registration approval from COEFPRIS?
No, a pharmaceutical product cannot be advertised before receiving a registration approval from COFEPRIS and doing so may cause legal consequences.