- Home
- >
- South Korea
- >
- Cosmetics
Cosmetics Product Registration and Regulatory Support in South Korea
South Korea MFDS Cosmetics Registration Services
Artixio is a regulatory consulting company with presence in over 100+ countries and providing our clients with complete support throughout their products’ lifecycle journey. With our regulatory experts team, we offer effective strategies by reducing the time to launch a product in the market and maintaining post approval activities.
Cosmetics Regulations in South Korea
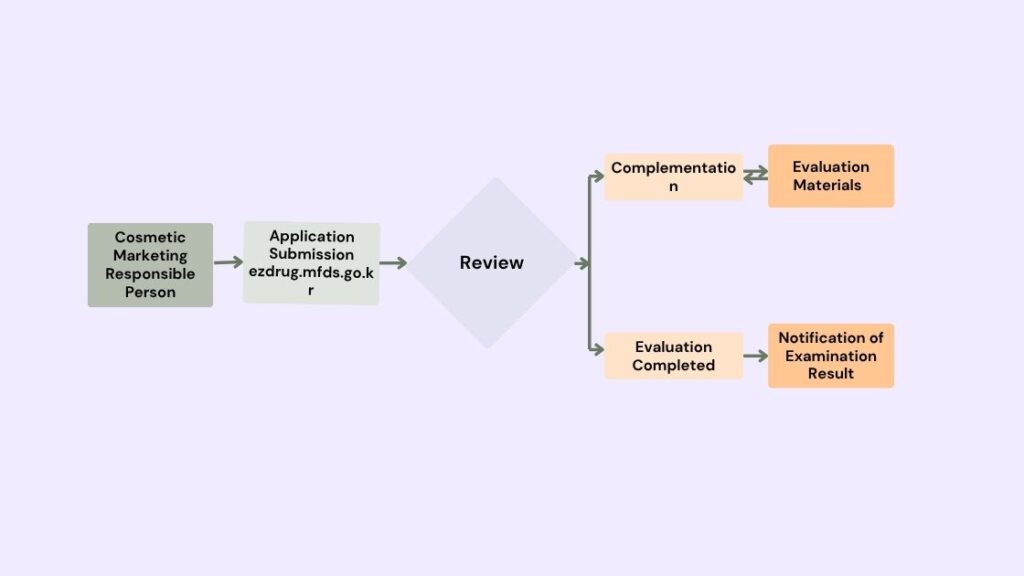
A responsible seller who intends to manufacture or sell functional cosmetics by manufacturing or importing them must undergo an evaluation by the Minister of the Ministry of Food and Drug Safety (MFDS) or must submit a report to the Minister of the MFDS for safety and effectiveness of each product.
Data Requirements for Submission
Data Verifying Safety, Effectiveness, or Function
- Data concerning the origin and details of R&D
- Data concerning the safety
- Data that verifies effectiveness, or function
- Sun Protection Factor (SPF), waterproof Sun Protection Factor (SPF), and Protection Factor of UV-A (PA) data
Data concerning the standards and test methods (including samples)
Artixio Offers the Following Services
- Strategic pre-registration guidance according to MFDS requirements
- Guidance for Successful registration of the product in South Korea Market
- Easy market access strategies by reducing the time to product launch
- Handling Health Authority queries
- Post-Approval maintenance of the product
Full Regulatory Support for Market Entry into South Korea
Why Choose Artixio For Cosmetic Product RA Services in South Korea





FAQs
What is the review time for evaluation of cosmetics by South Korea?
What are the fees to request evaluation of functional cosmetics in South Korea?
Electronic civil petitions – 189,000 KRW
By mail, in-person – 210,000 KRW
KRW: South Korean Won
Do you provide documentation services for cosmetic product registration in South Korea?
Related South Korea Services
Regulatory Expertise Across
Multiple Countries












Blogs

Orphan Drug Designation in
Orphan Drug Designation (ODD) is regulated by Ministry of Food and Drug Safety (MFDS)...

Biologics and Biosimilars Regulations
The Korean pharmaceutical market is estimated to be worth $24.3 billion in 2019, and...

Nutraceutical Regulation and Registration
Today, we eat to ease hunger and don’t have the time to consider what...