- Home
- >
- South Korea
- >
- Nutrition
Nutrition Product Registration and Regulatory Services in South Korea
End-to-End strategic regulatory service provider for Nutrition industry in South Korea. We support product registration and regulatory submissions in South Korea for food, dietary supplements, and health supplements. Our team works closely with MFDS to meet local compliance requirements.
Health and Dietary Supplement Registration – MFDS Korea
Artixio is a trusted and leading global regulatory service provider company. With our global experts team and integrated solutions, we help our clients to successfully launch their product by reducing the time to market entry. We provide end-to-end services for nutrition products in the South Korea including product branding and launching strategies, product registration in compliance with regulatory requirements of South Korea, and market strategy as well.
South Korea Food Product Registration & Regulatory Guidance
In South Korea, the Standards and specifications for Health functional food, was established to set criteria for manufacturing processing, production, import, distribution and preservation of health functional food products.
Approval of Functional Ingredients for Health Functional Food
- It shall be complied with the Health Functional Food Act; and
- Safety and functionality shall be ensured and scientifically proved.
Product Registration Approval Process
for Food and Supplements in Korea
The approval procedure for functional ingredient with the MFDS, South Korea is as follows:
- The applicant must submit application to Korea Food and Drug Administration (Korea FDA) to get the functional ingredient approved and notified in South Korea market.
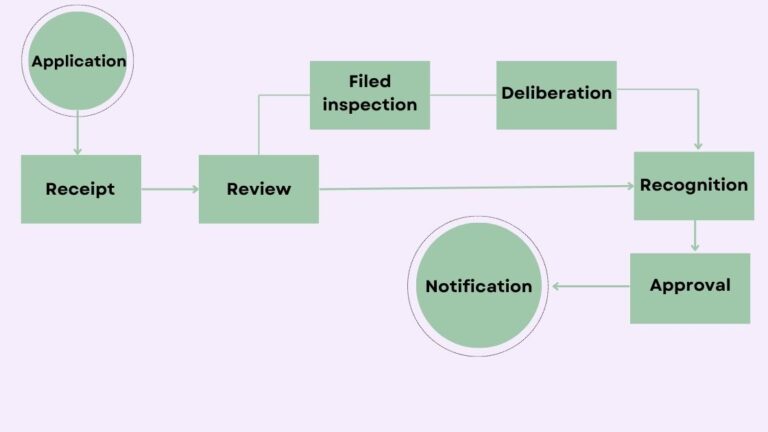
Registration of Foreign Food Facilities in South Korea
Applicant must online application through the Imported Food Information Maru
Registration Procedure Follows the Following Steps
- Application
- Receipt of documents
- Review
- Approval
- Notification of registration
Full Regulatory Support for Market Entry into South Korea
South Korea Regulatory Support for Nutrition & Supplements
- Stepwise guidance approval of a nutrition product in South Korea
- Strategies for compliance with MFDS requirements
- Support throughout product lifecycle
- Product registration and notification requirements
- Global regulatory support
Why Choose Artixio For Nutrition Product RA Services?






FAQs
What is the approval timeline for functional ingredient in South Korea?
Is there a provision by FDA to modify the contents of the certificate of functional ingredient? If yes, what is the procedure for it?
What is the applicable fees for approval application of Functional Ingredient for Health Functional Food?
What is the processing time for application of modification on Certificate of Functional Food Ingredient for Health Functional Food?
Related South Korea Services
Regulatory Expertise Across
Multiple Countries












Blogs

Orphan Drug Designation in
Orphan Drug Designation (ODD) is regulated by Ministry of Food and Drug Safety (MFDS)...

Biologics and Biosimilars Regulations
The Korean pharmaceutical market is estimated to be worth $24.3 billion in 2019, and...

Nutraceutical Regulation and Registration
Today, we eat to ease hunger and don’t have the time to consider what...





















