- Home
- >
- Veterinary
- >
- Regulatory Affairs
Regulatory Affairs Services for Veterinary Products
Artixio’s expertise has led to over 35 successful veterinary product approvals. Our streamlined regulatory services and in-depth knowledge empower our clients to achieve efficient and accelerated market entry.

Services We Offer
Artixio’s expertise has led to over 35 successful veterinary product approvals. Our streamlined regulatory services and in-depth knowledge empower our clients to achieve efficient and accelerated market entry.
Regulatory Affairs Services for Veterinary Products
- Artixio holds a comprehensive knowledge base of global and regional veterinary product regulations, covering a range of products including veterinary medicine, animal feed additives, diagnostics, devices, etc.
- We apply clear regulatory affairs strategies for veterinary medicines and follow a systematic approach for dossier preparation, submissions, and communication with regulatory authorities worldwide.
It is essential to obtain approval for veterinary products to ensure responsible and ethical use of veterinary products, failing which the consequences can be severe, impacting animal health, public safety, and the company’s reputation and financial stability.
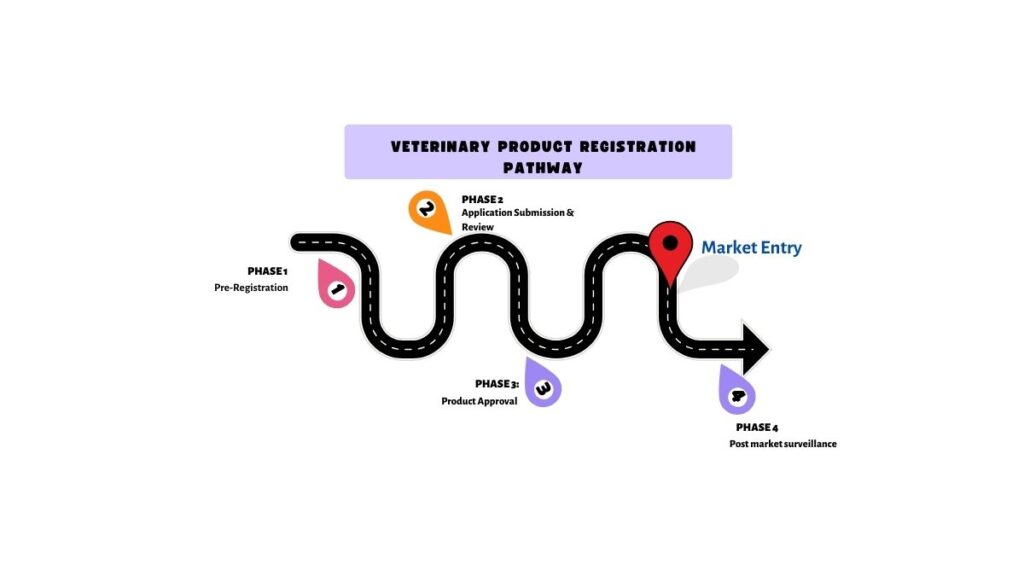
Veterinary Product Registration Pathway
Pre-registration
- Veterinary Regulatory Compliance and Strategy
- Prioritization of Animal Health Products
- Veterinary Market Research
- Animal Health Product Dossier Review and Gap Analysis
- Due Diligence of Veterinary Product
- Veterinary Product Market Access Strategy
Registration
- Animal Health Product Registration and Liaison
- Veterinary Product Dossier Preparation
- Animal Health Product Label and Packaging Review
- Veterinary Health Authority Meetings and Liaison
- Veterinary Health Authority Audit Support
- Veterinary eCTD/CTD Publishing and Submission
Post-Registration
- Veterinary Product Lifecycle Management
- Veterinary Product Label, Packaging, and Advertising Material Review
- Veterinary Product Line Extensions and Variations
- Response to HA Regulatory / Safety Queries
- Response to Veterinary Health Authority Regulatory and Safety Queries
- Veterinary Product Distribution and Import Support
Artixio’s Core Competencies
We offer consulting services for veterinary regulatory affairs backed by strong relationships with global regulatory bodies, including the US FDA, EMA, and other international authorities. This allows us to communicate effectively and help speed up the approval process.
We are a customer-first company, and believe in collaborating with the customer at every step throughout our journey. We provide tailor-made solutions for every specific regulatory requirement of our clients.
From concept to post-market surveillance, we offer end-to-end regulatory affairs support for veterinary products
Artixio’s comprehensive approach ensures regulatory considerations are integrated at every step of the veterinary product lifecycle management. Don’t let the regulatory hurdles slow you down. Partner with Artixio for your seamless product approvals, contact Artixio today at info@artixio.com
Why Choose ArtiXio For Veterinary Product RA Services?





FAQs
How can a robust regulatory strategy contribute to our company's overall business objectives for our veterinary product?
We believe that it directly contributes to your business objectives by reducing development costs, strengthening investor confidence, and providing a competitive edge.
What metrics does Artixio use to measure the success of a veterinary regulatory strategy?
What are some of the biggest challenges companies face in global veterinary regulatory affairs?
What strategies does Artixio employ to minimize the risk of regulatory delays for our veterinary product approvals?
How does Artixio help us manage the increasing data requirements for veterinary product registrations?
Still Have Questions ?
Specialized Registration Services Across Multiple Industries
Expert Regulatory Services To Streamline Compliance

Regulatory Intelligence & Strategy

Medical & Technical Writing

Publishing & Submission






Regulatory Affairs Across Multiple Countries

India

Singapore

Mexico

Brazil

Vietnam

Malaysia

Argentina

Colombia

Taiwan

China

China

Thailand

Indonesia

Philippines

USA

Japan

Qatar

South Korea
Insights from Artixio - Tips & Articles

HSA Guidance on Class
Advanced medicines, such as cell, gene, and tissue-engineered therapies, are revolutionizing modern medicine. In...

HSA Singapore Investigational Product
For locally registered products in Singapore, the management of investigational product (IP) for clinical...

Strategic Criteria for Selecting
All industries in Thailand, including cosmetics, food, medical devices, and nutrition, develop very fast,...