India’s medical device market is growing quickly and is now the fourth largest in Asia. The country is pushing to make more devices locally, aiming for better quality and safety. Anyone making or importing devices needs to understand CDSCO medical device regulations in India to avoid delays or compliance issues. These regulations can seem confusing at first. It can be confusing at first. Approvals, imports, manufacturing — there’s a lot to keep track of. But once you get the hang of it, moving your device through the system isn’t so bad.
Read on to find out how India’s medical device rules affect manufacturers and importers.
Regulatory Body For Medical Devices In India
Every medical device sold or made in India needs someone to keep an eye on the rules. That’s where CDSCO comes in. It works under the Ministry of Health & Family Welfare and is India’s official regulator for medical devices. People also call it the National Regulatory Authority.
If you’re planning to sell a device here, it helps to know the CDSCO guidelines for medical devices. This also ties into medical device registration in India, which is the process you’ll follow to get your product approved and into the market.
The CDSCO, an Indian medical device regulator, has a separate Medical Devices Division that is responsible for the regulation of medical devices. CDSCO Medical Devices Division is responsible for the following activities:
- Classification of medical devices
- India Medical Device Registration
- Import license for medical device in India
- Import of medical devices
- Manufacturing of medical devices
- Inspection of medical device manufacturing facilities
- Enforcement of medical device regulations including post market surveillance
Contact us for Medical Devices Regulatory & Registration Services in India
CDSCO MD Online Registration:
If you want to register a medical device in India, CDSCO MD Online is the first place to go. You fill in your details online and track what’s happening. It’s not complicated, but you’ll see there are a few rules to follow. Using the portal helps you stick to the CDSCO medical device regulations in India. It shows what steps you need to take, so you don’t get stuck along the way.
Classification of Medical Devices in India
The CDSCO has classified medical devices into four categories based on their risk to human health. The registration requirements for medical devices vary depending on their classification. The classification for the same is described below:
| Class | Risk-Level | Examples |
| Class A | Low risk | Gloves, hot water bags, etc. |
| Class B | Low-Moderate risk | Syringes, surgical instruments, etc. |
| Class C | Moderate-High risk | Infusion pumps, X-ray machines, etc. |
| Class D | Very high risk | Cardiac stents, pacemakers, etc. |
CDSCO Medical Device Regulation:
The CDSCO medical devices regulations in India are mainly regulated by the following laws:
- Drugs and Cosmetics Act, 1940 and Rules, 1945
- Medical Devices Rules, 2017
- Medical Devices (Amendment) Rules, 2020
- Gazette Notification S.O. 648(E)
- National Medical Devices Policy 2023
- Indian Pharmacopeia standards
CDSCO Medical Devices Legal Requirements:
Below are some of the legal requirements explained for medical devices at different steps:
1. Medical device manufacturing plant legal requirements:
- Manufacturers upon submission of the Plant Master File (PMF), will be granted licenses for the manufacturing of medical devices.
- The manufacturing unit must possess ISO 13485 certification.
- Every manufacturing site under a single manufacturer needs a separate manufacturing license.
2. Novel medical device requirements:
- Novel medical devices are known as “investigational devices” and hence they require more clinical investigations.
- A meeting is organized by the CDSCO called the Subject Expert Committee (SEC) meeting to discuss the investigational report of the novel device and address queries.
3. Medical device import requirements:
- Except all class A non-measuring non-sterile devices, all medical devices require an import license that is Form MD-15 for importation of devices in India.
- The import license that is Form MD-15 is obtained by submitting the application for the same through Form MD-14.
- Documents such as cover letter, regulatory certificates, device and plant master file, fee receipt, power of attorney, product and manufacturing authorizations, etc. are required to be submitted along with the import license application (Form MD-14).
- The applicant or manufacturing company, who holds a wholesale license (Form 21/21B) or registration certificate (Form 41/42) can apply for an import license, which is the prime requirement for importing a medical device in India.
- For foreign manufacturers in India, it is mandatory to appoint an Indian representative to carry out all the regulatory procedures.
4. BIS Regulations for medical devices:
- BIS investigates medical device-related products, especially the electronic category devices.
- Any medical device that comes under the category of electronic products needs BIS certification for the electronic medical device.
If a medical device is found to be non-compliant with the CDSCO’s regulations, the CDSCO may take several actions such as:
- Issuing a warning to the manufacturer or importer
- Directing the manufacturer or importer to recall the device
- Cancelling the registration of the device
- Taking legal action against the manufacturer or importer
Medical Device Registration in India
If you make or import a medical device in India, you have to get it registered. CDSCO takes care of this, and it’s their way of making sure everything is done right before the device can be sold.
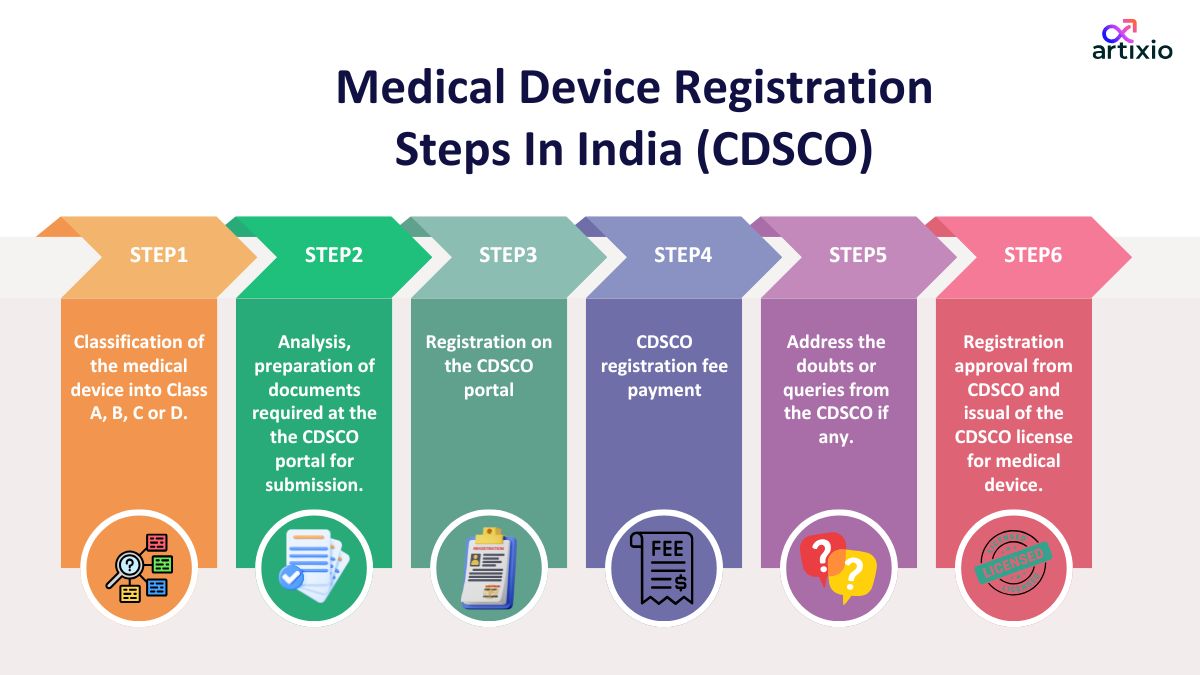
The following are the steps for medical device registration in India:
- Classification of medical device: In the first step the medical device is classified into one of the four classes of the medical device based on the risk level associated with the medical device.
- Preparation of documents required for CDSCO submission: In the second step all the required documents are prepared and reviewed for submission. The documents include the certificate of incorporation of the company, the free sale certificate from the country of origin, and the certificate of analysis from recognized laboratories, etc.
- Registration on CDSCO portal: In the third step the applicant registers on the CDSCO online portal.
- CDSCO registration fee payment: In the fourth step after the filling of the application the registration fee payment for the medical device is done.
- Addressing the doubts and queries from CDSCO: In the fifth step, if any doubts or queries from the CDSCO are addressed.
- Grant of CDSCO license for medical devices: After successful completion of all the above steps, in the sixth step the applicant is granted the approval license of the CDSCO for the sale of medical devices which is valid for five years and needs to be renewed before its expiry.
Also Read: CDSCO Medical Device Approval Cost
CDSCO Medical Device Form Numbers For New Medical Devices:
| Form No | Purpose of Application | Type of License |
| Application: MD-22 Permission: MD-23 | For clinical investigation of new or modified medical device | Clinical Investigation Permission (For Importer or domestic manufacturer) |
| Application: MD-26 Permission: MD-27 | For registration and importation of new medical devices in India, especially which are not yet notified for regular import | Import license (For Importer applicant) |
| Application: MD-16 Permission: MD-17 | License to import medical devices for testing purposes. | Test license (For Importer applicant) |
| Application: MD-3 Permission: MD-5 | License to manufacture medical devices in India. | Manufacturing license for Class A and B |
| Application: MD-4 Permission: MD-6 | Loan manufacturing License to allow one company to produce medical devices on behalf of another. | Loan Manufacturing license for Class A and B. |
| Application: MD-7 Permission: MD-9 | Loan for manufacturing arrangements. | Loan Manufacturing license for Class C and D. |
CDSCO Medical Device Form Numbers For Existing Medical Devices:
| Form No | Purpose Of Application | Type of License |
| Application: MD-14 Permission: MD-15 | Registration of imported medical devices | Importer License for Importer |
| Application: MD-3 Permission: MD-5 | Manufacturing License to manufacture medical devices in India | Manufacturing License for Class A and B |
| Application: MD-4 Permission: MD-6 | Loan License to manufacture medical devices on behalf of other company | Loan License for Class A and B |
| Application: MD-7 Permission: MD-9 | Manufacturers applying for licenses to produce certain types of medical device | Manufacturing License for Class C and D |
| Application: MD-8 Permission: MD-10 | Loan License agreement applications for medical devices | Loan License for Class C and D |
| Application: MD-18 Permission: MD-19 | Registration of new medical devices by manufacturers. | Registration license for Class C and D |
| Application: MD-22 Permission: MD-23 | Distribution License for medical devices. | Distribution license for Class A and B |
It is important to note that the medical device registration process in India can be complex and time-consuming. It is advisable to consult with a qualified consultant or professional to help you with the registration process.
Here are some additional tips for medical device registration in India:
- Make sure that the medical device complies with all the Indian regulatory requirements, including labeling and GMP requirements.
- Gather all of the required documents before submitting the registration application.
- Be prepared to answer any questions that CDSCO may have about the medical device or the registration application.
- Work with a qualified consultant or professional to help you with the registration process.
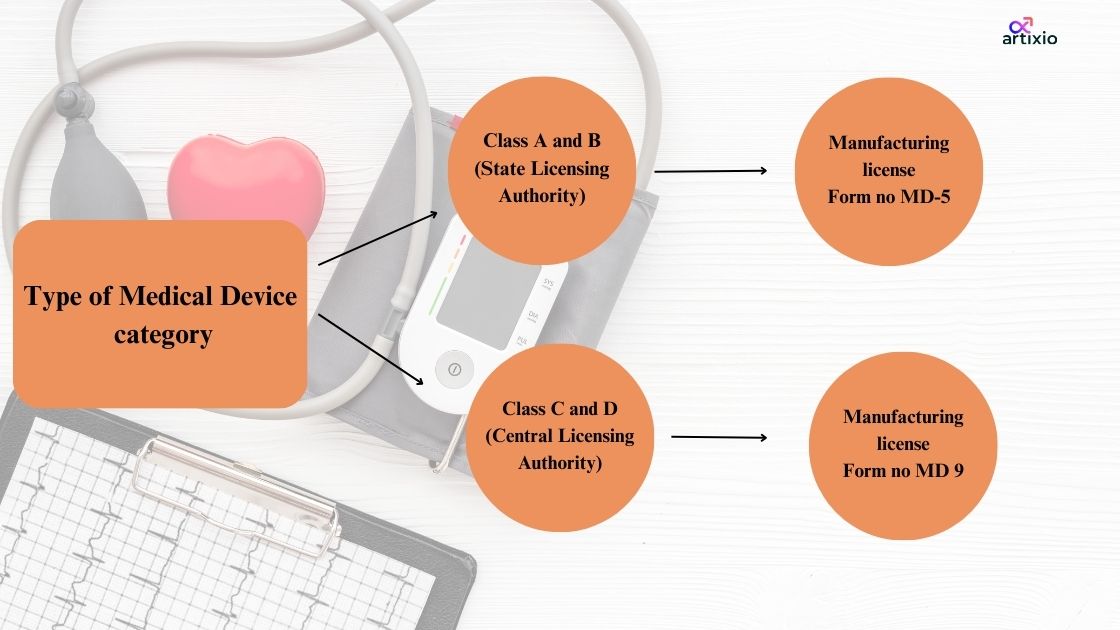
All About CDSCO Manufacturing License:
- The manufacturer must have the manufacturing license under the Indian medical device regulation.
- He should apply for the same through CDSCO’s online portal that is SUGAM, where he should fill the online application based on the device’s classification.
- The application is then inspected by the CDSCO team, and if the application is found to be authentic the CDSCO grants the manufacturing license which is valid for 5 years.
- The type of form to be filled in for the manufacturing license application depends upon the category of your medical device.
Below is the illustration of how the form number is decided according to the medical device category:
All About CDSCO Import License:
- The manufacturers who intend to import their medical devices to India is required to obtain the import license under the Indian medical device regulation.
- To obtain this license the manufacturers should fill Form MD 14 and Form MD 15 on the SUGAM online portal.
- The validity of this license is for a period of 5 years.
Role Of Indian Authorized Agent (IAA) In Medical Device Compliance:
- For foreign medical device manufacturers who intend to export their devices in India require a local Indian Authorized Agent to handle all their regulatory compliance in India.
- Hence the compliance agent plays a vital role in the journey of foreign medical devices approval and market access in India.
- The foreign manufacturer must hire an authorized agent on a contractual basis for their regulatory journey in India.
- It is the compliance agent who is responsible for the entire approval process, product compliance, document compliance, product recalls, any non-compliance, coordination with the CDSCO, etc.
- Therefore, while selecting the authorized agent it should be checked that he has accurate knowledge and experience of the regulatory strategies.
Challenges In Meeting Device Compliance And Maintaining It:
Some of the challenges faced by meeting the Device Compliance are as follows:
Advances and Updates in the regulations:
There are continuously being advances and updates in the regulations which require constant updates with the new advances for up-to-date compliance.
Cyber-security:
As nowadays everything is updated and stored on the computer system, any technical issue or viruses may lead to the loss of essential documents and data which may cause delays in timely compliance.
Collaboration gap:
Any miscommunication in the collaboration team may lead to minor or major errors which may eventually cause non-compliance.
Inaccurate Documentation:
Any mistake in the documentation process for the medical device may lead to excessive time in compliance. This is essential to comply in time with all the required, correct and authentic documents.
Partner Artixio For Medical Device Regulatory Compliance With CDSCO:
Working in India’s medical device market isn’t always simple. There are rules to follow, like the Medical Device Rules, device classifications, and quality standards. And these rules keep changing, so it’s easy to get stuck if you’re handling it alone.
That’s where Artixio comes in. We help you understand India medical device regulations by CDSCO, follow the CDSCO guidelines for medical devices, and complete medical device registration in India. We also make sure you meet all the medical device regulatory requirements in India.
If you want guidance on getting your devices approved and into the market, we can walk you through each step. You’ll know what’s needed and what to do next — making the whole process less stressful.
Contact us to get expert help with your
Combining Interna
FAQs:
How can a medical device be registered in India with CDSCO?
The medical device can be registered in India by filing the application on the CDSCO online portal and by attaching all the relevant documents with it. The whole process for medical devices should be in compliance with the Medical Device rules (MDR).
What is the CDSCO timeline required for medical device approval?
The time required for the approval of the medical device is generally several months, however it varies depending upon the class of medical device and also on the authenticity and relevancy of the documents and per-clinical and clinical data submitted. Any noncompliance may extend the approval timeline.
What is the role of Indian Authorized Agent (IAA) in the medical device compliance?
The IAA is responsible for the medical device compliance with CDSCO in India. He carries out all the regulatory process on behalf of the foreign manufacturer who intends to sale the medical device in India.
What are the penalties if the medical device fails to comply with the CDSCO?
The manufacturer and the compliance agent have to undergo certain penalties such as fines, product recalls, and market withdrawal or legal actions if the device fails to comply with CDSCO.